FIGURE 4.
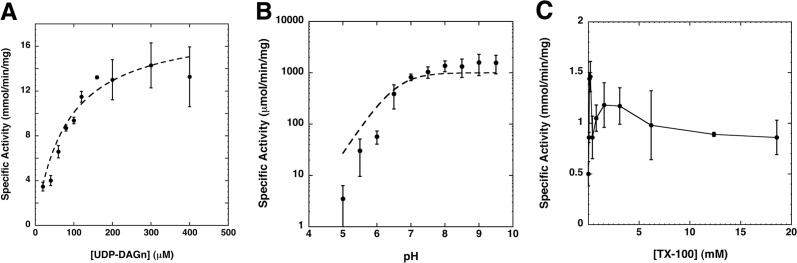
Enzymatic analysis of HiLpxH. A, HiLpxH activity under standard conditions is dependent upon UDP-DAGn concentration. The apparent Km was 79.4 ± 11.0 μm and the apparent Vmax was 18.1 ± 0.9 mmol min−1 mg−1, both calculated by fitting the data using KaleidaGraph. B, changes in pH alter the in vitro activity of HiLpxH. The enzyme exhibits a dramatic increase in activity as the pH of the reaction reaches 7 but very little change upon progressively more alkaline conditions. Data were fit to Equation 1 using KaleidaGraph, yielding a pKa of 6.56 ± 0.38. C, effect of Triton X-100 concentration on HiLpxH-specific activity at 100 μm UDP-DAGn. Surface dilution kinetics are not apparent as activity does not appear to have a strong correlation with detergent amount, and the enzyme displays detectable activity even at very low Triton X-100 (TX-100) concentrations. All plots shown were each generated from the average of three separate experiments.
