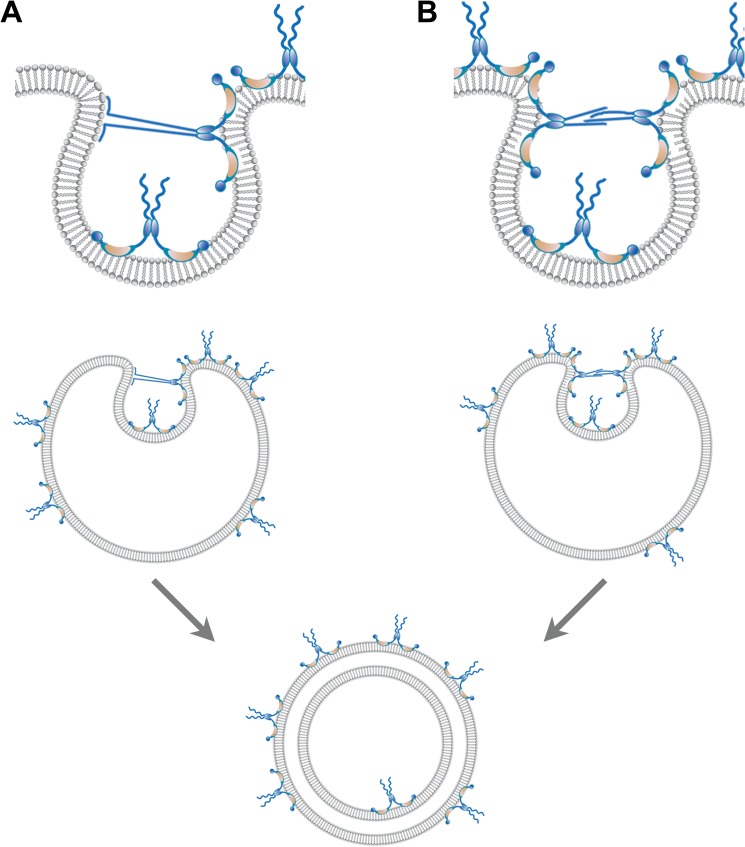
FIGURE 6.
Two hypotheses for the formation of double-membraned vesicles induced by poliovirus 3AB. The 3AB integral membrane protein inserts into the outer leaflet of the bilayer in a wedge-like fashion, with the N- and C-terminal domains both protruding from the cytoplasmic surface (25, 30). To promote membrane invagination, we propose that the 3AB protein dimers displace lipids in the outer leaflet, generating a localized deformation of the bilayer. This localized deformation leads to lateral crowding and an imbalance in the leaflets of the bilayer, stabilizing the curvature required for invagination. As more 3AB molecules associate with the vesicle surface, lateral crowding and stress increase until the protein-sparse region collapses inward. The neck of the invaginating structure is stabilized by interactions between the unstructured domains of 3AB either with (A) the negatively charged phospholipid head groups (A) or the unstructured domains of 3AB dimers (B) on opposite sides of the neck. It is likely that this close opposition, together with the membrane disruption, promotes fusion. Note that most 3AB molecules remain on the outside of the vesicle, as expected for the participation of 3AB in viral RNA replication. Random coil, unstructured N-terminal domain; blue oval, dimerization domain; orange crescent, hydrophobic domain; blue circle, C-terminal 3B domain.