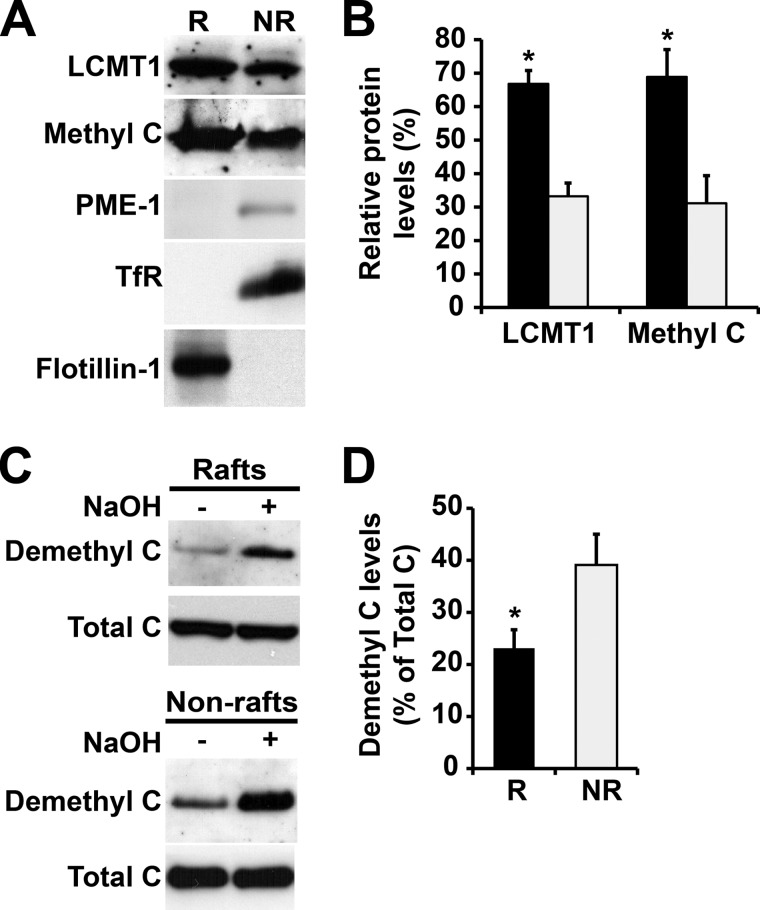
FIGURE 2.
Differential distribution of LCMT1, PME-1, and methylated and demethylated PP2A enzymes in N2a cell membrane microdomains. A, equivalent aliquots of proteins (∼15 μg) from pooled R and NR plasma membrane fractions purified from N2a cells were analyzed by SDS-PAGE followed by Western blotting for the presence of LCMT1, PME-1, and methylated PP2A C subunit (Methyl C). B, note the preferential enrichment of LCMT1 and methylated PP2A in R (black bars) versus NR (gray bars) fractions (n = 4, mean ± S.D.; *, p < 0.001, R versus NR). The levels of PME-1 were too low to be quantified. C, the distribution of demethylated PP2A was assessed with a specific monoclonal anti-demethyl C antibody in the same purified R and NR fractions. Parallel treatment of duplicate aliquots of R and NR fractions with sodium hydroxide (+ NaOH) induces complete demethylation of PP2A, thereby allowing for detection of total C subunit levels with the same antibody. Note that NaOH treatment does not affect the distribution of total C in R and NR fractions, as detected by a methylation-insensitive antibody. D, quantification of the total percentage of PP2A in a demethylated form in R and NR fractions (n = 4, mean ± S.D.; *, p < 0.001, R versus NR). Note the preferential concentration of demethylated C enzymes in NR fractions.