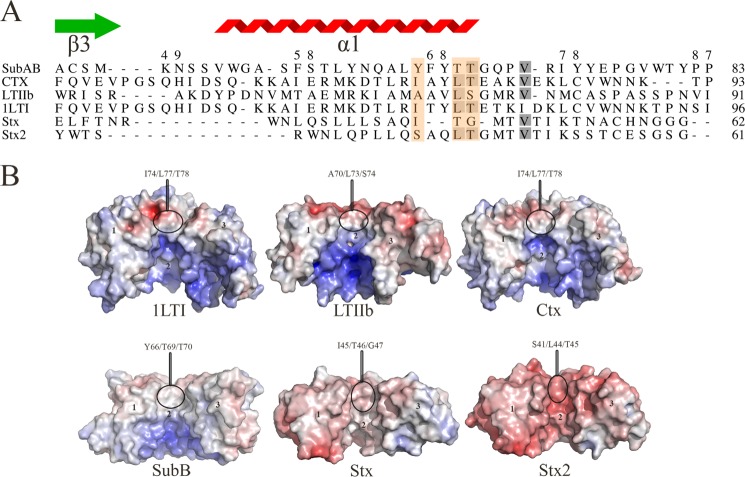
FIGURE 5.
A, structure-based sequence alignment of the B-subunit α1-helix of members of the four AB5 toxin families. The residues that form the hydrophobic patch are boxed in orange. The gray shadowing indicates the sequence identity level between the six sequences. The SubB secondary structural elements are shown. The figure was generated using the Indonesia program (16). B, calculated molecular electrostatic surface potential of members of the four AB5 toxin families (SubAB, LT-I, LT-IIb, Ctx, Stx, and Stx2). For clarity, the surface of three protomers for each AB5 toxins (1 to 3) is shown. The molecular electrostatic surface potential was calculated as described in the legend for Fig. 4. The figure was generated using PyMOL (29).