FIGURE 3.
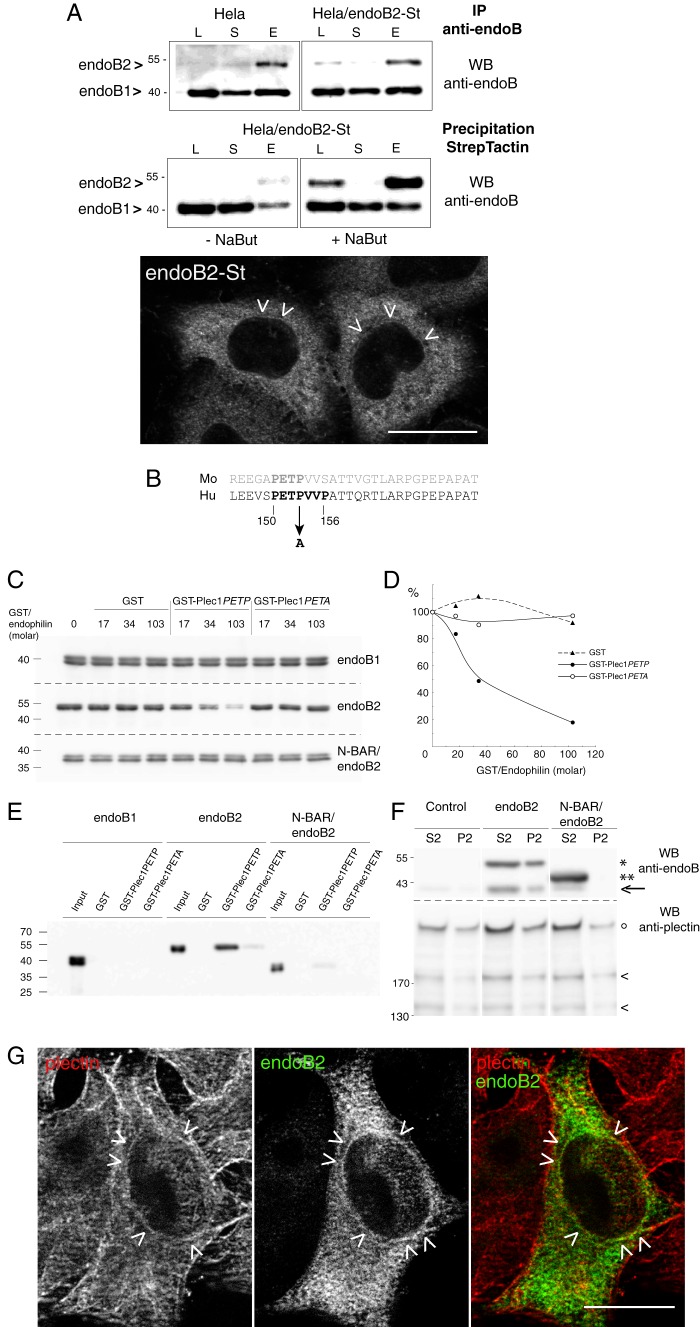
Plec1 is a molecular partner of endoB2 SH3 domain. A, HeLa/endoB2-St, a stable cell line aimed at isolating in situ pre-formed complexes with endoB2 binding partners. Top two panels, Western blot (WB) analysis of lysates (L), supernatants (S) of precipitations, or eluates (E) from either immunoprecipitations (IP) on protein A-Sepharose (upper panel) or StrepTactin/Sepharose precipitations (lower panel) from HeLa or HeLa/endoB2-St cell lines as indicated (note that no background from beads was detected, neither for the protein A-Sepharose immunoprecipitations nor for the StrepTactin-Sepharose precipitations). Western blots were treated with anti-Bif-1 antibody recognizing both endoB2 and endoB1. Calibration curves using pure recombinant bacterial endoB1 and endoB2 showed that this antibody recognizes endoB1 with ∼10 times more affinity/avidity than endoB2. Detection of endoB2 in the lysates from either HeLa or HeLa/endoB2-St cells is stochastic, and in most experiments, immunoreactivity is undetectable unless HeLa/endoB2-St cells are treated with 5 mm sodium butyrate (compare in lower panel, −NaBut and +NaBut). From the immunoprecipitations (upper panel), it was estimated that the expression level of endoB2-St in HeLa/endoB2-St cells compares with endoB2 level in HeLa cells. From the StrepTactin-Sepharose precipitations (lower panel) and taking into account the 10-fold difference in anti-Bif-1 immunoreactivities mentioned above, it was calculated that a maximum of 40 or 2% of endoB2 is in a complex with endoB1 in the absence (− NaBut) or the presence (+ NaBut) of sodium butyrate, respectively. Bottom panel, immunodetection of endoB2-St in HeLa/endoB2-St cells treated overnight with 5 mm sodium butyrate and using an anti-StrepTag antibody. Arrowheads point at discrete immunoreactivity around nuclei (see also G). Note in addition the homogeneous staining that appears mainly cytosolic. Scale bar, 20 μm. B, alignment of mouse and human plec1 sequences (human amino acid residues 145–174) showing the putative PETP consensus motif for SH3 domain binding. The mutation (at position 153) is indicated to illustrate the difference between GST-plec1PETP and GST-plec1PETA. C, in vitro binding assay of endophilin B species to immobilized GST, GST-plec1PETP, and GST-plec1PETA (see under “Experimental Procedures”). Three molar ratios of GST chimera to endophilin were tested as indicated. The immunoblots of unbound fractions obtained from separate acrylamide gels are shown, with endophilin revealed by anti Bif-antibody. GST/endophilin 0 refers to control sample performed in the absence of GST or chimera. D, depletion curves of the unbound fraction as determined from the experiment illustrated in C. Chemiluminescence signals are plotted as a percentage of the value determined for GST/endophilin 0 unbound fraction (equivalent to the input material). E, immunoblot analysis of material bound on beads and corresponding to samples analyzed in C, with GST/endophilin molar ratio of 103. F, specific coimmunoprecipitation of plectin and endoB2 from HeLa cells. Cells transfected with the indicated two endoB2 species (or mock-transfected) were processed as described under “Experimental Procedures.” Immunoblots of fractions P2 and S2 are shown (similar results were obtained in two independent experiments). In the upper panels, the arrow points at the faint band corresponding to endogenous endoB1 visible in fractions S2 of the control and comigrating with an endoB2 degradation product visible in the two endoB2 lanes, full-length (*) and truncated (**) endoB2 species. In lower panels showing material with molecular mass above 130 kilodaltons, intact plectin (°) and two of its major proteolytic degradation products (<) are distinguished. G, z-section obtained from HeLa/endoB2-St cells treated overnight with 5 mm sodium butyrate and processed for double immunofluorescence analysis using anti-StrepTag and anti-plectin antibodies. Arrowheads point to discrete colocalization of endoB2 and plectin around nuclei. Scale bar, 20 μm.