FIGURE 4.
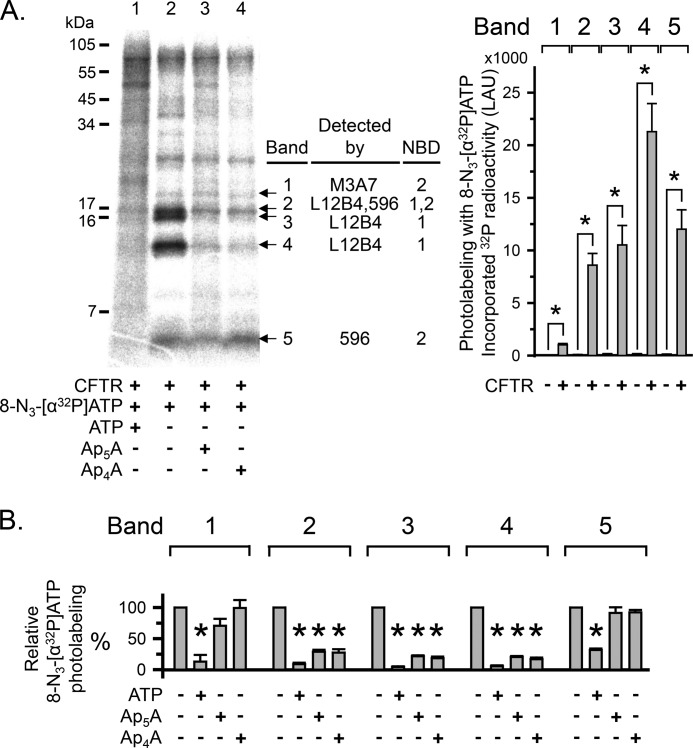
Ap5A and Ap4A do not interact with all CFTR ATP-binding sites. A, left, autoradiograph. Photolabeling of CFTR with 50 μm 8-N3-[α-32P]ATP was performed as described in the legend to Fig. 3. 5 mm non-radioactive ATP, Ap4A, or Ap5A were present as indicated below the lanes of the autoradiograph. After photolabeling, CFTR was solubilized, immunoprecipitated, and subjected to partial proteolysis with the proteinase Arg-C. The digestion products were fractionated on a 16% Tricine gel. To compare with undigested photolabeled and immunoprecipitated CFTR, see Fig. 3. Middle, summary of Western blot analysis results (see Fig. 5). Right, quantitative data in linear arbitrary units (LAU) for radioactivity incorporated into the different CFTR fragments (bands 1–5) after labeling with 8-N3-[α32P]ATP. *, p ≤ 0.024 compared with background control of no CFTR present (Mann-Whitney rank sum test, n = 3–8). B, quantitative data for photolabeling bands 1–5 with 50 μm 8-N3-[α-32P]ATP in the presence or absence of 5 mm non-radioactive ATP, Ap4A, or Ap5A. Amount of radioactivity incorporated into each band was normalized to radioactivity for control conditions (i.e. absence of non-radioactive ATP, Ap4A, or Ap5A). *, p ≤ 0.001 (one-way repeated measures ANOVA followed by Holm-Sidak's method of multiple comparisons versus control group, n = 2–5). Error bars, S.E.