FIGURE 9.
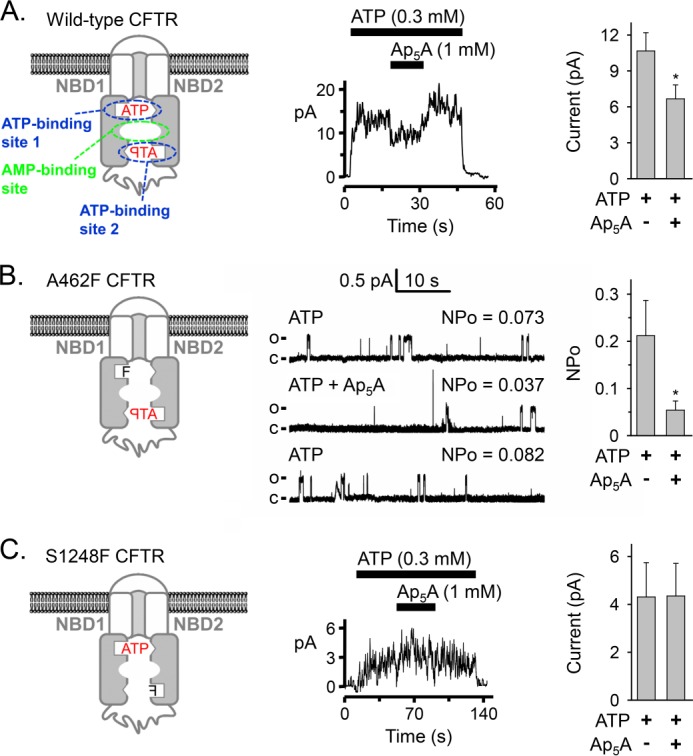
Effect of phenylalanine mutations in the phosphate-binding loop (Walker A motif) of either ATP-binding site on Ap5A inhibition of CFTR current. A, left, model of CFTR. The phosphate-binding loops are depicted as open rectangles, and the ABC signature motifs are shown as open triangles. The binding site for AMP is not known. Middle, current recording (100 ms averages) from an excised inside-out membrane patch containing multiple CFTR channels. ATP and Ap5A were present during the times and at the concentrations indicated by bars. ATP was added together with PKA catalytic subunit. Holding voltage was −50 mV. Right, CFTR Cl− current before and after adding 1 mm Ap5A. Experiments were performed as shown in the middle panel with 0.3 mm ATP and PKA present. *, p < 0.001 (Wilcoxon signed rank test, n = 13 paired experiments of current measurements before and after adding Ap5A obtained from five membrane patches). B, left, model of A462F CFTR. Middle, current recording from one excised inside-out membrane patch containing at least two A462F CFTR channels perfused on cytosolic surface with ATP and Ap5A as indicated. PKA catalytic subunit was present throughout the recording. Holding voltage was −50 mV. Each lane shows 48 s of recording and mean NPo. The first lane shows the trace in the presence of 0.3 mm ATP, the second lane shows the recording after 1 mm Ap5A was added, and the third lane shows the recording after Ap5A was removed again. For illustration purposes, traces were digitally low pass-filtered at 50 Hz. c, channel closed state; o, single channel open state. Right, NPo of A462F CFTR with 0.3 mm ATP and PKA present in the bath solution before and after adding 1 mm Ap5A. *, p = 0.002 (Wilcoxon signed rank test, n = 10 membrane patches). C, left, model of S1248F CFTR. This mutant contained an N-terminal 6-histidine tag between CFTR amino acids 2 and 3. Middle, current recording (100 ms averages) from an excised inside-out membrane patch containing multiple S1248F CFTR channels. ATP and Ap5A were present during the times and at the concentrations indicated by bars. ATP was added together with PKA catalytic subunit. Holding voltage was −80 mV. Right, S1248F CFTR Cl− current before and after adding 1 mm Ap5A. Experiments were performed as shown in the middle panel with 0.3 mm ATP and PKA present. No significant differences were detected (p = 0.463, Wilcoxon signed rank test, n = 14 paired experiments of current measurements before and after adding Ap5A obtained from four membrane patches). Error bars, S.E.
