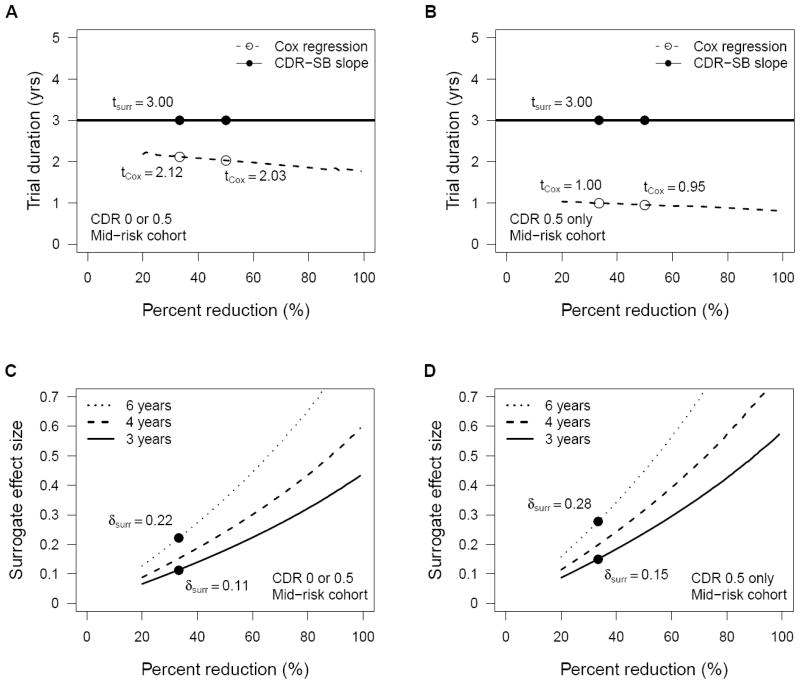
Figure 3.
Trial duration (A and B) and minimum surrogate treatment effects (C and D) required for 80% power in all non-demented subjects (A and C) and CDR 0.5 only non-demented subjects (B and D) populations. Estimates assume 1:1 randomization, one year accrual, and two-tailed testing at alpha = 0.05.