Abstract
Simultaneous PET/MRI is an emerging technique combining two powerful imaging modalities in a single device. The wide variety of available tracers for perfusion and metabolic studies and the high sensitivity of positron emission tomography (PET) combined with the high spatial resolution and soft tissue contrast of magnetic resonance imaging (MRI) in depicting cardiac morphology and function as well as MRI's absence of ionizing radiation makes PET/MRI very attractive to radiologists and clinicians. Nevertheless, PET/MR scientific and clinical promise is to be considered in the context of numerous technical challenges that hinder its use in the clinical setting. For example, in order for a PET system to work correctly within an MR field, major changes are required to the photon detection chain such as the elimination of photomultiplier tubes, etc. Another significant limitation of PET/MRI is the lack of an electron density map (as is the case with PET-CT) that can be readily obtained from MRI (the latter measures proton not electron density) and used to correct PET data for attenuation. Moreover, as with PET-CT, cardiac and respiratory motions cause image degradations that affect image quality and accuracy both in static and dynamic PET imaging. As a result, overcoming these (and other) technical limitations is a very active area of research both in academic institutions as well as industry. In this paper, we review recent literature on cardiac PET/MRI, present the state-of-the-art of this technology, and explore promising preclinical and clinical cardiac applications where PET/MRI could play a substantial role.
Keywords: PET MRI, Hybrid PET/MRI, Cardiovascular disease, Coronary artery disease, Ischemia
Introduction
Although the cardiovascular discipline is among the most studied areas of medicine, heart disease remains the leading cause of death in the U.S. and in the Western World and early detection of coronary artery disease (CAD) remains key to patient outcome. In this context, multimodal imaging is an attractive approach to investigate, potentially in a one-stop-shop, structure, anatomy and function of the heart.
PET-computed tomography (PET-CT) is the modality of choice to assess perfusion, metabolism and regional absolute myocardial blood flow (MBF), allowing the characterization of the epicardial and small vessel function that is involved in the early steps of the ischemic cascade [1]. PET-CT however has several limitations including limited spatial resolution (typically ~4–6 mm), misregistration of attenuation (CT) and emission (PET) images due to spatial as well as temporal mismatch between CT and PET, yielding artifactual false positive myocardial perfusion defects [2]. Furthermore, PET has been reported to underestimate the extent and severity of ischemia [3]. Cardiac MRI has become the clinical gold standard for assessment of cardiac chamber volume and myocardial mass.
Furthermore, diffusion MRI methods [4] have been developed to map myocardial architecture in vivo and late gadolinium contrast enhancement (LGE) allows detection of defects in the subendocardium and differentiatiation of non-transmural and transmural myocardium infarcts [5]. Moreover, MRI offers several benefits when evaluating heart failure hence allowing scar identification (LGE), fat delineation (T1 weighted black blood with fat suppression), tissue edema and inflammation imaging (T2-weighted sequences). It becomes clear that simultaneous PET and MRI acquisition has great potential to yield crucial complementary data that is key clinically for patient management. Furthermore, there is potential to improve the information content of one modality with the other (e.g., MR-measured organ motion used to correct PET data) [6•]. Thus, forasmuch as MR imaging is a serial image technology and PET is a fully volumetric approach, the opportunity of acquiring data from both modalities at the same time under the same cardiac conditions opens a promising opportunity to collect comprehensive information in a one-stop-shop setting [7••]. Other potential advantages offered by combined PET/MRI in the investigation of cardiovascular disease, include complementary assessment of wall motion abnormalities and identification of regional and segmental hypokinesis, as well as wall thinning, scar tissue and a correlation of microcirculation with delayed contrast-enhanced MR and tissue characterization. Moreover, the absence of radiation exposure and the higher soft tissue contrast represent significant advantage of hybrid PET/MRI over PET/CT in a cardiac rest stress scan [8]. Furthermore, MR perfusion imaging, without use of contrast agents, allows detection of cardiac disease in patients with renal failure that can not undergo PET/CT [9]. We will present in this paper state-of-the-art imaging that can be obtained with the new promising hybrid PET/MRI systems, and touch upon the clinical scenarios where such system could play a role in the clinic.
Technological Considerations
A major challenge encountered in simultaneous PET/MRI system design pertains to the major effects that the magnetic field has on electrons traveling in vacuum inside the photomultiplier tubes (PMTs), that transform the visible light produced in the scintillator into an electric signal detectable by PET electronics. This is a key reason why, despite continuous efforts to build PET/MRI systems even before PET/CT, combining PET and MRI for clinical studies revealed a challenge for which solutions have been proposed [10]. Currently there are three commercially available PET/MR devices, two of which acquire PET and MR data sequentially and one simultaneously. The sequential hybrid imaging system from Philips (The Ingenuity TF PET/MR Philips Healthcare, Cleveland, OH) [11] that integrates the two modalities using a turntable between MRI and PET; The sequential trimodality PET/CT-MRI scan consists of two major components: a 3T MRI system (Discovery 750w 3T, GE Healthcare, Waukesha, WI, USA) and a state-of-the-art TOF PET/CT (Time of flight, Discovery 690, GE Healthcare, Waukesha, WI, USA) [12] in adjacent rooms connected by shuttle bed; The fully integrated PET/MRI system (Siemens Biograph mMR) [13] simultaneously acquires PET and MRI data.
Several detector designs have been proposed. The most advanced system, using avalanche photodiode (APD) detectors, is not sensitive to magnetic fields [14]. This detector technology proposed in 2008 [15], provides an integrated PET/MRI system that can simultaneously acquire PET and MRI data. Simultaneous PET/MRI scans provide several benefits. The high time resolution motion information from MRI (e.g., tagged GRE sequence, true FISP sequence) provides natural spatial and temporal alignment between the two modalities and with high spatial resolution and high signal-to-noise ratio (SNR) [16]. Chun et al. have recently reported on novel MRI-based non-rigid motion correction schemes in simultaneous PET/MRI [17] that improve PET image quality, especially in cardiac imaging, and are very promising in clinical cardiac applications. The latter case, tagged MRI-based motion correction does not require additional time (it is clinically indicated to measure wall motion) nor exposure to ionizing radiation while resolving the ambiguity in iso-intense tissues associated with conventional methods examining temporal changes in MRI or CT image intensity [17].
Although intrinsic spatial resolution of modern whole-body (WB) PET scanners is in the 4–6 mm range for stationary objects [18–22], inevitable cardiac and respiratory motions (the heart moves during free breathing in the range of 5–10 mm), substantially deteriorate this resolution [22, 23]. Moreover even though cardiac and/or respiratory gating strategies that “freeze” motion are popular in cardiac PET, they have not been effective in dynamic PET imaging of rapidly changing functions, such as myocardial blood flow (MBF), due to the substantial increase in noise associated with rejecting a large number of detected events in a short time frame. These factors have a significant role in image degradation and in reducing the accuracy of MBF quantification. Finally, partial volume effect (PVE) reduces SNR and introduces bias in measured myocardial activity concentration, especially for non transmural infarcts [6•].
Therefore, an approach that independently measures the 4D (3D+t) motion field from MR during PET studies would greatly improve spatial resolution without the reduction in SNR associated with cardiac and respiratory gating.
Tagged MRI imposes a spatially periodic magnetization pattern (tags) before image acquisition, using a combination of radiofrequency pulses and field gradient pulses. After the tagging pulses, the magnetization pattern persists over a specified evolution time and is distorted by motion, which can be quantified in the MR images. Spatial modulation of magnetization creates a sinusoidal modulation of magnetization in space by exciting (and subsequently dephasing) an interference pattern in the transverse magnetization due to a pair of rectangular “hard” radiofrequency pulses separated by a gradient in time [24]. The magnetization pattern moves with the tissue, revealing positional changes occurring between the tagging preparation pulses and the image acquisition. Cyclic processes such as cardiac contraction and breathing can be modeled as periodic, thus allowing the image to be formed over multiple cycles [18].
Preclinical Applications
Cardiovascular preclinical research is one of the most active fields due to its challenging and promising nature, and is one where PET/MRI has great potential to play a key role in understanding structure and function. Büscher et al. [24] successfully used a prototype hybrid PET/MR to assess cardiac parameters in a mouse infarct model, although performance was reduced when compared with results from a high-resolution animal PET scanner. More recently, Lee et al. [25] explored myocardial inflammationpost infarction in mice with coronary ligation using 18F-Fluorodeoxyglucose (18F-FDG) PET/MRI system. They devised a fusion method combining separate scanners. Mice were imaged by microPET/CT using an Inveon (Siemens) small animal scanner and a 7-T BrukerPharmascan with electrocardiogram and respiratory gating (SA instruments). PET/MR fusion was facilitated by a “framed fusion” approach implemented by using external fiducial landmarks. Gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) enhanced infarcts showed high 18F-FDG uptake on day 5 after myocardial infarction and the number of monocytes/macrophages in the non infarcted myocardium was lower than in the ischemic tissue. This evidence suggested that infarct signal on day 5 after ischemia reflects inflammatory activity despite 18F-FDG - limitations when imaging inflammation. In fact, this is a potential avenue for research that involves radiotracers specific for inflammation (e.g., 18F -FDG, 64Cu nanoparticles, 68Ga labeled peptides).
These experiments are initial attempts exploring how PET/MRI technological development can finally meet an increasing demand from research to combine valuable data from the most advanced modalities in cardiac diagnostic imaging.
As detailed above, respiratory and cardiac motions represent serious limitation to what spatial resolution can be achieved (~ 1cm) with cardiac PET. Furthermore, motion-induced inconsistencies in the attenuation measurements often lead to significant artifacts in the reconstructed images. Specifically, motion can cause a portion of the myocardium in PET perfusion study to fall in the corresponding lung area of the CT yielding a reduced recovery of intensity on PET that is often mistaken for a hypoperfusion in the PET scan (false positive). Respiratory and cardiac gating can remove motion artifacts, but at the cost of increased noise. Chun et al. have recently reported on a novel approach to respiratory motion correction using simultaneous PET/MR in beating phantoms, rabbits and nonhuman primates [17]. MRI-based motion correction in simultaneous PET/MR showed significant improvements in lesion detection. This method increased lesion contrast, SNR and image quality, compared with the conventional respiratory and cardiac gated approach, without increasing background noise or acquisition time. This result is promising for whole body imaging and, even more so, for cardiovascular imaging where cardiac and respiratory motion play a determinant role in the image accuracy and quality of cardiac PET perfusion and/or metabolism [26].
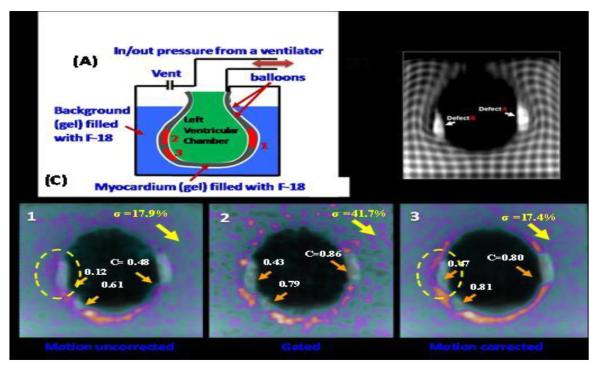
Heart motion measurement is challenging compared to other organs as it has three separate components: motion caused by the pumping action of the four chambers (cardiac motion), motion caused by respiration, and voluntary/involuntary patient movement. The latter component is usually managed by patient cooperation or using sedation or anesthesia in pediatric patients. The relationship between the motion of the heart and the superior inferior (SI) motion of the diaphragm due to breathing is approximately linear, although highly subject specific, with an element of hysteresis [26]. Regarding the cardiac motion component, Petibon et al. [6•] developed a list-mode iterative reconstruction framework incorporating both tagged-MR derived non rigid myocardial wall motion and position dependent detector point spread function (PSF) directly into the PET system matrix. This method was evaluated in a cardiac beating non-rigid phantom, which mimics cardiac motion (Fig. 1). This phantom, with small transmural (Gd MR enhancing) and non-transmural cold defects inserted in the hot myocardial compartment, was constructed and scanned using simultaneous PET/MR. The proposed method yielded better defect/myocardium contrast recovery as compared to no PSF modeling by about 8.5% improving heart wall reconstruction and quantitative image accuracy [6•]. These results were confirmed in vivo in pigs who were instrumented to achieve proximal left anterior descending artery (LAD) occlusion. An 18F -BMS747158-02 [27] PET-MR scan was performed 4 weeks after LAD occlusion to allow for recovery. Motion uncorrected, gated and motion corrected methods for reconstruction were compared. Figures 2 and 3, demonstrate the improvement in myocardial wall uptake by 25–35% when using MR-based motion correction as compared to conventional static and gated cardiac studies. These results demonstrate the feasibility of cardiac motion compensation thereby addressing one of the most challenging aspects of non-invasive imaging: the evaluation and quantification of the presence and severity of ischemia.
Fig. 1.
Deformable cardiac beating phantom. (A) Schematic design. (B) Coronal slice with tagging. (C) Coronal slice of reconstructed PET image for motion uncorrected, gated, motion corrected. Defect contrast C and noise σ computed over 15 noise realizations are shown (arrows). Note clean separation of MR (Late Gadolinium Enhanced, LGE) and PET (perfusion) in C.3 but not in C.1 and C.2 (circle)
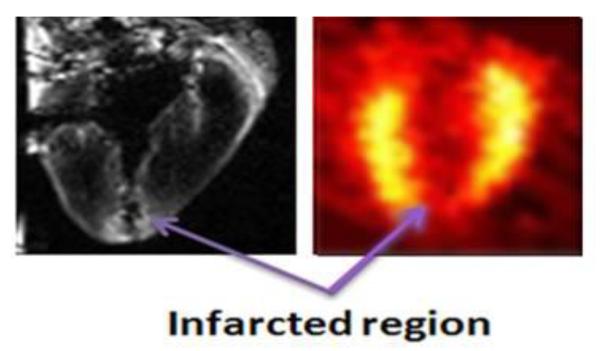
Fig. 2.
Chronic porcine infarct study using simultaneous PET-MR. Late Gadolinium Enhanced (LGE) MR (left) and PET perfusion (right) images of infarcted myocardium. Infarcted region are bright on the LGE image and dark (hyposignal) on the PET perfusion image. The dark section running through the apex LGE image is a section of resected tissue that was sampled to perform pathological assessments of the infarcted region
Hybrid PET/MR may be particularly useful for following molecular and cellular events after myocardial infarction. Majmudar et al. [28] reported on labeling a macrophage-targeted nanoparticles with 64Cu and imaging mice using PET/MR three days after myocardial infarction. Delayed enhancement after injection of gadolinium-DTPA delineated the infarcted myocardium, while PET reflected inflammatory cells that incorporated the nanoparticles. They hypothesized that this signal could represent a key wound-healing process - removal of necrotic tissue - and may be a potential predictor outcome. The concept could be expanded further by adding a molecular MRI agent to track two biologic targets simultaneously. Furthermore, they highlighted [29] the advantages of combining PET and MRI in detection of macrophages in atherosclerotic plaques, using hybrid PET/MR in apolipoproteinE knockout mice. They used dextran nanoparticles (DNP) labeled with desferoxamine to chelate zirconium-89. Although PET and MRI were done sequentially and data were fused offline, they demonstrated the feasibility of nanoparticle-facilitated hybrid PET/MR of inflammatory leukocytes in murine atherosclerotic plaques, suggesting a potential future application for newer scanners that allow simultaneous PET and MR data acquisition.
Clinical Applications
Clinical assessment of cardiovascular disease can strongly benefit from simultaneous PET/MR scanner. Sensitivity and specificity in coronary artery disease detection is about 90% for PET imaging and 91% and 81% respectively for MR [30, 31]. Combined PET/MR offers a number of potential advantages in the investigation of cardiovascular disease, including complementary assessment of wall motion abnormalities and identification of regional and segmental hypokinesis, as well as wall thinning and scar tissue. As a result, PET can identify hibernating myocardium, while LGE with MR can assess acute myocardial infarction or scarring at the site of prior infarction.
Moreover, the correlation of microcirculation by absolute quantification of MBF by PET with delayed contrast-enhanced MR and tissue characterization could prove crucial when investigating micro vascular disease and differentiating between microvascular dysfunction and epicardial stenosis, especially in cardiometabolic patients [32]. Several PET tracers are available today to investigate cardiac viability and perfusion.
18F FDG is the metabolic marker used most commonly in PET imaging for myocardial viability. FDG is a cyclotron-produced glucose analog that undergoes positron emission with a half-life of 110 minutes. Perfusion radiotracers such as 13N-ammonia, 82Rb Rubidium and 15O-water are clinically established for cardiac studies. All three radiotracers have short half-lives: 76 seconds for 82Rb, ten minutes for 13N-ammoniaand 2.1 minutes for 15O-water, which makes consecutive measurements of myocardial blood flow at rest and during hyperemic feasible in the same visit [33].
Currently, two promising 18F -labeled tracers for myocardial perfusion are under validation in clinical trials: 18F -flurpiridaz (formerly known as 18F -BMS747158-02) and which binds to the mictochondrial complex within the myocytes and 18Ffluorobenzyltriphenylphosphonium (FbnTP), that is based on a lipophilic cation, which can cross biological membranes by passive diffusion and it is concentrated within intact mitochondria [33, 34]. Since cardiomyocytes are rich in mitochondria, these tracers accumulate to a high degree in the heart.
Berman et al. [34] reported initial results with relative quantitation of 18F -flurpiridaz PET images acquired from patients enrolled at Cedars-Sinai Medical Center and UCLA in the phase 2 multicenter 18F flurpiridazstudy. The reported results are promising in terms of image quality and regional count distribution. These finding have been confirmed by several groups including ours, and confirmed in porcine models with absolute myocardial blood flow validated by microsphere methodology in simultaneous PET/MR pig studies. 18F -NaF is a promising new tracer as well, especially for the assessment of coronary artery plaque physiology [35]. Dweck et al. [36] recently found in a retrospective study that patients with increased coronary 18F-NaF activity had higher rates of prior cardiovascular events and angina, and higher Framingham risk scores. Prospective studies with clinical outcomes are now needed to assess the diagnostic value of coronary 18F-NaF uptake. Noninvasive hybrid PET/MR imaging of inflammation and angiogenesis within atherosclerotic lesions may be useful to predict future risk of plaque rupture and allow monitoring of antiatherosclerotic therapies [37].
Coronary artery disease and myocardial ischemia and infarction are not the only potential cardiac clinical applications of hybrid PET/MR. A preliminary study of cardiac imaging in active cardiac sarcoidosis was reported by O'Meara et al. [38]. Cardiac sarcoidosis is a potentially fatal complication of sarcoidosis. 18F-FDG PET is not currently included in the diagnostic guidelines of sarcoidosis. However, several studies have shown promising results using 18F-FDG PET. Youssef et al. [39] reported recently in a systematic review, that 18F-FDG PET yielded 89% sensitivity and 78% specificity in the diagnosis of cardiac sarcoidosis and O'Meara et al. reported in a case study the promising role of simultaneous PET/MRI in differentiating between active and chronic cardiac sarcoidosis [38].
Ibrahim et al. [40] have recently reported initial work in simultaneous 18F-FDG PET/MRI to identify sustained regional abnormalities in cardiac metabolism and function in stress induced transient mid-ventricular ballooning syndrome, which is a variant of Takotsubo cardiomyopathy (TTC). The combined information of integrated PET/MR in this condition allowed physicians to delineate neurogenic myocardial stunning. The latter is yet another clinical indication where PET/MR can provide additional insight into the underlying pathophysiology of stress-induced cardiomyopathy.
Counter-Indications, Challenges and Opportunities
A major limitation of PET/MR systems is related to the unavoidable presence of an electromagnetic field in MR. Therefore, patients with pacemakers, implantable cardioverter defibrillators or mechanical heart valves are excluded from any MR diagnostic imaging, as well as from any MR cardiac imaging. Nevertheless, PET/MR allows to halve the total radiation dose associated with PET studies by eliminating the CT component. This, however, comes at a price: the lack of an electron density map (as is the case with PET-CT) that can be readily obtained from MRI (the latter measures proton not electron density) in order to correct PET data for attenuation. This limitation is being addressed using several strategies. One approach is to use atlas registration [41–45]. In order to obtain the attenuation map for an acquired MR image, a reference CT image can, in principle, be registered to the MR image using deformable inter-modality registration. Alternatively, a reference MR image, previously registered to a reference CT image, can be first registered to the MR image using deformable intra-modality registration followed by the same transformation of the reference CT image. Another approach, which may have the potential for clinical use, is to segment an MRI image into different tissue types and then assign the corresponding attenuation coefficients to them [43, 46–49].
Another challenge of clinical cardiac PET-MR pertains to the interpretation of the PET and MR studies. The interpretation of PET/MRI scans requires a new generation of well-trained specialists in both PET and MRI expertise [50]. However, the increased acceptance of this technology presents a unique opportunity where physicians would be encouraged to garner the specific skills needed for the use of cardiac PET/MRI in the clinical setting.
Another challenge for PET/MRI compared to PET/CT is the increased patient compliance that is required with PET/MRI, especially during breath-hold acquisitions. Nevertheless motion correction sequences present a unique opportunity to overcome these limitations. Furthermore, the opening of PET/MRI bores is only 70-cm in diameter. This size limits the patient population that would benefit from PET/MR. Nevertheless; the medical imaging industry is currently on wider bore scanner that would mitigate this limitation. Finally, cost remains a major limitation that has hindered the widespread use of PET/MR as compared to PET/CT (the price of PET/MR scanners is roughly double that of PET/CT scanners). Therefore, even if the number of installed scanners is increasing rapidly, it is not yet enough to guarantee routine clinical use on a large scale and additional targeted clinical studies need to be done to better understand the clinical scenarios where PET/MRI will impact patient management and identify the real potential of this technology. If the ongoing studies confirm the usefulness of PET/MRI in performing an early and detailed cardiac assessment, this new technology will overcome the sensibility and sensitivity limitations of available diagnostic imaging methods by helping prevent unnecessary clinical and surgical procedures that contribute to rising healthcare costs and negatively impact patient outcomes.
Conclusions
We have discussed the state-of-the-art and the potential preclinical and clinical applications of hybrid PET/MRI systems in cardiac diagnostic imaging. Limitations of PET/MRI associated with the lack of attenuation maps directly usable in PET reconstruction were detailed and potential solutions discussed using atlas registration and image segmentation. The promising potential of simultaneous PET/MRI in addressing one of the major factors affecting image quality and accuracy in PET, i.e., cardiac and respiratory motion was detailed and recent results in this area discussed and shown to be very promising in achieving the full potential of cardiac PET in terms of spatial resolution, image quality and accuracy of myocardial blood flow quantitation. PET/MRI emerges as a powerful tool with great potential in the evaluation of suspected or known coronary artery disease, in the assessment of myocardial viability and microvascular disease and in estimation of risk of plaque rupture. Other potential clinical applications include investigation of inflammatory response following myocardial infarction and therapy guidance of left ventricle (LV) remodeling in heart failure. Ultimately, the “proof is in the pudding”, and it is hoped that the increasing number of installed PET/MRI scanners will not only contribute to increase acceptance of PET/MRI in the clinical setting, but also, identification of the key applications where PET/MRI will bring unique information, unavailable otherwise.
Fig. 3.
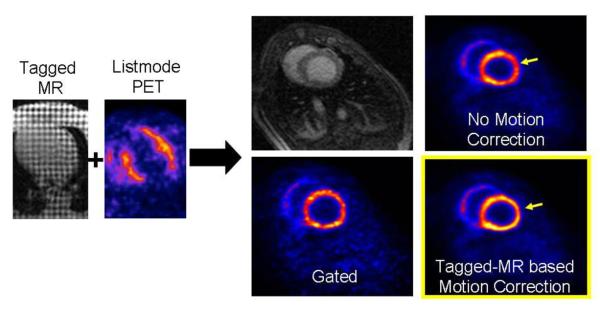
Simultaneous perfusion PET and tagged MR porcine study. Tagged MR (left) and listmode reconstruction of PET perfusion images of an infarcted pig myocardium (left). Short axis MR slice and motion uncorrected, gated, and motion corrected short-axis PET slices (right). As compared to the motion uncorrected method, the motion correction method improves the myocardium uptake by 25–35%. Also, the motion correction method dramatically reduces the image noise as compared to the gated method
Acknowledgments
The authors would like to acknowledge research funding from the National Institutes of Health (NHLBI-HL110241, PI: Dr El Fakhri) and SDN Foundation (PI: DrNappi). The views presented in this work do not reflect the position of SDN or NIH.
Footnotes
Conflict of Interest Carmela Nappi declares she has no potential conflict of interest. Georges El Fakhri declares he has no potential conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59(7):23C–30C. doi: 10.1016/0002-9149(87)90192-5. [DOI] [PubMed] [Google Scholar]
- 2.Loghin C, Sdringola S, Gould KL. Common artifacts in PET myocardial perfusion images due to attenuation-emission misregistration: clinical significance, causes, and solutions. J Nucl Med. 2004;45(6):1029–39. [PubMed] [Google Scholar]
- 3.Di Carli MF, Dorbala S, Hachamovitch R. Integrated cardiac PET-CT for the diagnosis and management of CAD. J Nucl Cardiol. 2006;13(2):139–44. doi: 10.1007/BF02971234. [DOI] [PubMed] [Google Scholar]
- 4.Dou J, et al. Cardiac diffusion MRI without motion effects. Magn Reson Med. 2002;48(1):105–14. doi: 10.1002/mrm.10188. [DOI] [PubMed] [Google Scholar]
- 5.Miller SW, B.L., Abbara S. The requisites- Cardiac Imaging. 3rd edn. Elseviers Mosby; 2009. ISBN-13: 978-0323055277 2009. [Google Scholar]
- 6•.Petibon Y, et al. Cardiac motion compensation and resolution modeling in simultaneous PET-MR: a cardiac lesion detection study. Phys Med Biol. 2013;58(7):2085–102. doi: 10.1088/0031-9155/58/7/2085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Good paper about a novel method for cardiac motion compensation in a list-mode iterative reconstruction framework in simultaneous PET-MR.
- 7••.Rischpler C, et al. Hybrid PET/MR Imaging of the Heart: Potential, Initial Experiences, and Future Prospects. J Nucl Med. 2013;54(3):402–15. doi: 10.2967/jnumed.112.105353. [DOI] [PubMed] [Google Scholar]; Great review of hybrid PET/MRI application in heart disease.
- 8.Koepfli P, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med. 2004;45(4):537–42. [PubMed] [Google Scholar]
- 9.Zhang H, et al. Accurate myocardial T1 measurements: toward quantification of myocardial blood flow with arterial spin labeling. Magn Reson Med. 2005;53(5):1135–42. doi: 10.1002/mrm.20461. [DOI] [PubMed] [Google Scholar]
- 10.Delso G, Ziegler S. PET/MRI system design. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S86–92. doi: 10.1007/s00259-008-1008-6. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi H, et al. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56(10):3091–106. doi: 10.1088/0031-9155/56/10/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veit-Haibach P, et al. PET-MR imaging using a tri-modality PET/CT-MR system with a dedicated shuttle in clinical routine. MAGMA. 2013;26(1):25–35. doi: 10.1007/s10334-012-0344-5. [DOI] [PubMed] [Google Scholar]
- 13.Delso G, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52(12):1914–22. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 14.Judenhofer MS, Cherry SR. Applications for preclinical PET/MRI. Semin Nucl Med. 2013;43(1):19–29. doi: 10.1053/j.semnuclmed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Judenhofer MS, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14(4):459–65. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 16.Prince JL, McVeigh ER. Motion estimation from tagged MR image sequences. IEEE Trans Med Imaging. 1992;11(2):238–49. doi: 10.1109/42.141648. [DOI] [PubMed] [Google Scholar]
- 17.Chun SY, et al. MRI-based nonrigid motion correction in simultaneous PET/MRI. J Nucl Med. 2012;53(8):1284–91. doi: 10.2967/jnumed.111.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stickel JR, Cherry SR. High-resolution PET detector design: modelling components of intrinsic spatial resolution. Phys Med Biol. 2005;50(2):179–95. doi: 10.1088/0031-9155/50/2/001. [DOI] [PubMed] [Google Scholar]
- 19.Sureau FC, et al. Impact of image-space resolution modeling for studies with the high-resolution research tomograph. J Nucl Med. 2008;49(6):1000–8. doi: 10.2967/jnumed.107.045351. [DOI] [PubMed] [Google Scholar]
- 20.Wiant D, et al. Evaluation of the spatial dependence of the point spread function in 2D PET image reconstruction using LOR-OSEM. Med Phys. 2010;37(3):1169–82. doi: 10.1118/1.3310381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessio AM, et al. Application and evaluation of a measured spatially variant system model for PET image reconstruction. IEEE Trans Med Imaging. 2010;29(3):938–49. doi: 10.1109/TMI.2010.2040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher L, et al. Respiratory gating for 3-dimensional PET of the thorax: feasibility and initial results. J Nucl Med. 2004;45(2):214–9. [PubMed] [Google Scholar]
- 23.Blume M, Navab N, Rafecas M. Joint image and motion reconstruction for PET using a B-spline motion model. Phys Med Biol. 2012;57(24):8249–70. doi: 10.1088/0031-9155/57/24/8249. [DOI] [PubMed] [Google Scholar]
- 24.Buscher K, et al. Isochronous assessment of cardiac metabolism and function in mice using hybrid PET/MRI. J Nucl Med. 2010;51(8):1277–84. doi: 10.2967/jnumed.110.076448. [DOI] [PubMed] [Google Scholar]
- 25.Lee WW, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59(2):153–63. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang J, Li Q, El Fakhri G. Magnetic resonance-based motion correction for positron emission tomography imaging. Semin Nucl Med. 2013;43(1):60–7. doi: 10.1053/j.semnuclmed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higuchi T, et al. Targeting of Endothelin Receptors in the Healthy and Infarcted Rat Heart Using the PET Tracer 18F-FBzBMS. J Nucl Med. 2013;54(2):277–82. doi: 10.2967/jnumed.112.106096. [DOI] [PubMed] [Google Scholar]
- 28.Majmudar MD, Nahrendorf M. Cardiovascular molecular imaging: the road ahead. J Nucl Med. 2012;53(5):673–6. doi: 10.2967/jnumed.111.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majmudar MD, et al. Polymeric Nanoparticle PET/MR Imaging Allows Macrophage Detection in Atherosclerotic Plaques. Circ Res. 2013;112(5):755–61. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker MW, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5(6):700–7. doi: 10.1161/CIRCIMAGING.112.978270. [DOI] [PubMed] [Google Scholar]
- 31.de Jong MC, et al. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol. 2012;22(9):1881–95. doi: 10.1007/s00330-012-2434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koller A, Balasko M, Bagi Z. Endothelial regulation of coronary microcirculation in health and cardiometabolic diseases. Intern Emerg Med. 2013;8(Suppl 1):51–4. doi: 10.1007/s11739-013-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rischpler C, et al. Advances in PET myocardial perfusion imaging: F-18 labeled tracers. Ann Nucl Med. 2012;26(1):1–6. doi: 10.1007/s12149-011-0552-5. [DOI] [PubMed] [Google Scholar]
- 34.Berman DS, Germano G, Slomka PJ. Improvement in PET myocardial perfusion image quality and quantification with flurpiridaz F 18. J Nucl Cardiol. 2012;19(Suppl 1):S38–45. doi: 10.1007/s12350-011-9487-4. [DOI] [PubMed] [Google Scholar]
- 35.Dweck MR, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59(17):1539–48. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Dweck MR, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125(1):76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 37.Ratib O, N.R. Cardiovascular clinical applications of PET/MRI. Clinical Translational Imaging. 2013 doi:10.1007/s40336-013-0008-0. [Google Scholar]
- 38.O'Meara C, M.L.J., White SK, Elliott P. Inital experience of imaging cardiac sarcoidosis using hybrid PET-MR - a technologist's case study. J Cardiovasc Magn Reson. 2013 doi: 10.1186/1532-429X-15-S1-T1. [Google Scholar]
- 39.Youssef G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53(2):241–8. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim T, et al. Simultaneous positron emission tomography/magnetic resonance imaging identifies sustained regional abnormalities in cardiac metabolism and function in stress-induced transient midventricular ballooning syndrome: a variant of Takotsubo cardiomyopathy. Circulation. 2012;126(21):e324–6. doi: 10.1161/CIRCULATIONAHA.112.134346. [DOI] [PubMed] [Google Scholar]
- 41.Rota Kops E, Herzog H. Alternative methods for attenuation correction for PET images in MR-PET scanners. IEEE NuclSciSympConf Rec. 2007;6:4327–4330. [Google Scholar]
- 42.Rota Kops E, Qin P, Muller-Veggian M, Herzog H. MRI based attenuation correction for brain PET images. Springer Proc In Phys. 2007;114:93–97. [Google Scholar]
- 43.Beyer T, et al. MR-based attenuation correction for torso-PET/MR imaging: pitfalls in mapping MR to CT data. Eur J Nucl Med Mol Imaging. 2008;35(6):1142–6. doi: 10.1007/s00259-008-0734-0. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann M, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med. 2008;49(11):1875–83. doi: 10.2967/jnumed.107.049353. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann M, et al. MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med. 2011;52(9):1392–9. doi: 10.2967/jnumed.110.078949. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg J, et al. Three-region MRI-based whole-body attenuation correction for automated PET reconstruction. Nucl Med Biol. 2010;37(2):227–35. doi: 10.1016/j.nucmedbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Moller A, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50(4):520–6. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 48.Schulz V, et al. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging. 2011;38(1):138–52. doi: 10.1007/s00259-010-1603-1. [DOI] [PubMed] [Google Scholar]
- 49.Eiber M, et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38(9):1691–701. doi: 10.1007/s00259-011-1842-9. [DOI] [PubMed] [Google Scholar]
- 50.Catana C, Guimaraes AR, Rosen BR. PET and MR Imaging: the odd couple or a match made in heaven? J Nucl Med. 2013 Mar 14; doi: 10.2967/jnumed.112.112771. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]