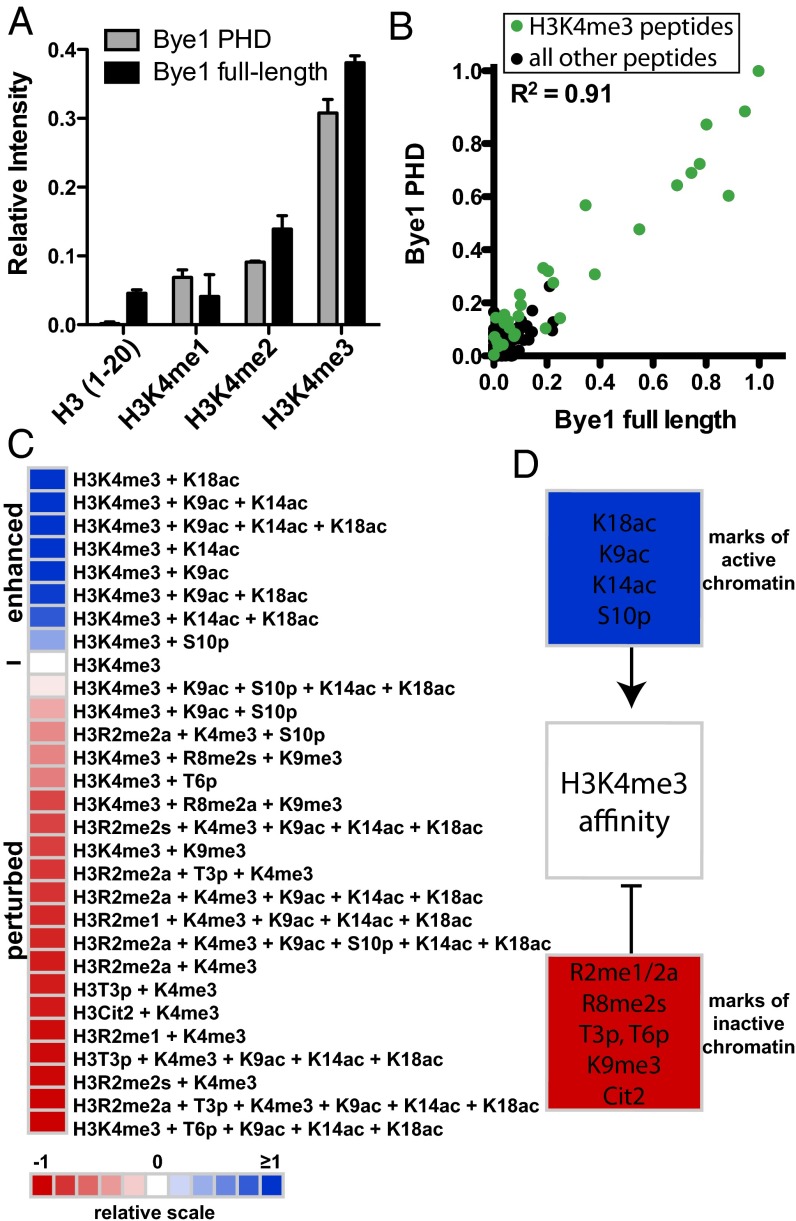
Fig. 4.
Bye1 preferentially binds histone peptides carrying PTMs of active transcription. (A) Peptide array binding analysis reveals that Bye1 preferentially associates with H3K4me3 peptides, and its PHD domain is sufficient for this interaction. Results of two independent arrays consisting of 24 individual spots for each peptide (Table S2) are presented as relative mean intensity measurements on a scale from 0 to 1, with 1 being the most significant peptide interaction. (B) Scatter plot correlating relative mean intensity measurements of all peptide interactions (Table S2) from arrays probed with full-length Bye1 and the Bye1 PHD domain. H3K4me3-containing peptides are shown as green dots. All other peptides on the array are shown as black dots. The correlation coefficient was calculated by linear regression analysis using GraphPad Prims v5. (C) Heat map depicting the effects of combinatorial PTMs on the binding of Bye1 to H3K4me3-containing peptides. Average binding intensities are represented relative to the H3K4me3 peptide (0, white). Enhanced (>0, blue) and perturbed (<0, red) interactions are depicted. (D) Summary of modifications enhancing (blue) and perturbing (red) the Bye1 interaction with H3K4me3 peptides.