Fig. 6.
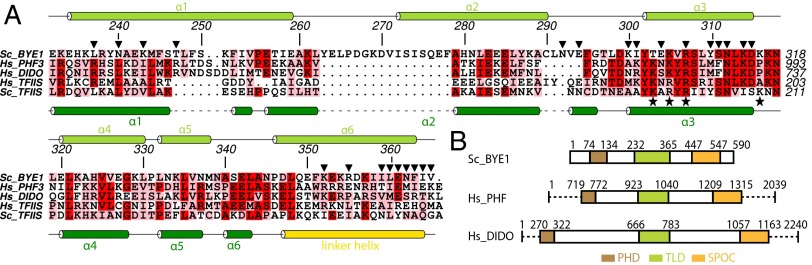
Conservation of Pol II–binding residues in Bye1 human homologs. (A) Amino acid sequence alignment of S. cerevisiae Bye1, H. sapiens PHF3, H. sapiens DIDO, H. sapiens TFIIS, and S. cerevisiae TFIIS. Secondary structure elements are indicated as arrows (β-strands) or rods (α-helices). Loops are indicated with solid lines. Residues that are part of the Pol II–Bye1 interface are marked with black triangles. Residues essential for the Pol II–TFIIS interaction (51) are marked with black asterisks. (B) Domain organization of S. cerevisiae Bye1, H. sapiens PHF3, and H. sapiens DIDO. Numbers for bordering residues are indicated.
