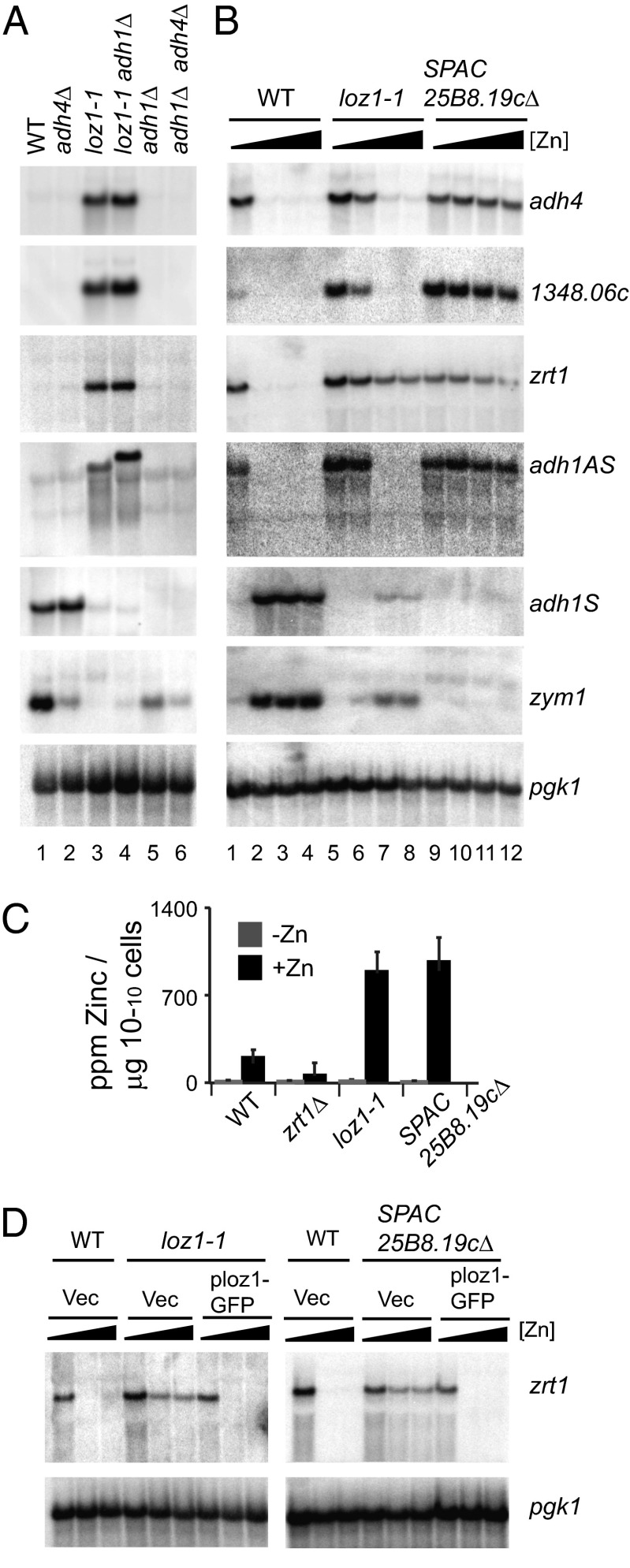
Fig. 1.
A mutation in loz1 disrupts zinc-dependent regulation of gene expression. (A) Wild-type, adh4Δ, loz1-1, adh1Δ loz1-1, adh1Δ, and adh1Δ adh4Δ cells were grown to exponential phase in YES medium and total RNA purified for RNA blot analysis. RNA blots were incubated with strand-specific probes that hybridized to the adh4, zrt1, SPBC1348.06c, adh1AS, adh1, and zym1 transcripts. The adh1AS probe hybridizes to the extreme 5′ end of this transcript, a region that does not overlap with the adh1 ORF and is present in adh1Δ cells. Because of the high level of sequence conservation at the DNA level (>99% identity), the SPBC1348.06c probe can cross-hybridize to SPAC977.05c and SPBPB2B2.15 transcripts. For a loading control, blots were also probed for pgk1. (B) Wild-type, loz1-1, and loz1Δ cells were grown in ZL-EMM supplemented with 0, 50, 200, or 500 μM zinc. Total RNA was purified for RNA blot analysis. RNA blots were probed as described in A. (C) Wild-type, zrt1Δ, loz1-1, and loz1Δ cells were grown in ZL-EMM supplemented with 0 μM (-Zn) or 200 μM zinc (+Zn). Cells were harvested, and total cellular zinc levels measured by inductively coupled plasma mass spectrometry. (D) Wild-type, loz1-1, and loz1Δ cells containing the vector JK148 or plasmid pLoz1-GFP were grown in ZL-EMM supplemented with 0, 100, or 200 μM zinc, and total RNA was purified for RNA blot analysis.