Abstract
Secreted frizzled related protein 2 (SFRP2) is overexpressed in human angiosarcoma and breast cancer, and stimulates angiogenesis via activation of the calcineurin/ NFATc3 pathway. There are conflicting reports in the literature as to whether SFRP2 is an antagonist or agonist of ß-catenin. The aims of these studies were to assess the effects of SFRP2 antagonism on tumor growth and Wnt-signaling, and to evaluate whether SFRP2 is a viable therapeutic target. The anti-angiogenic and anti-tumor properties of SFRP2 monoclonal antibody (mAb) were assessed using in vitro proliferation, migration, and tube formation assays; and in vivo angiosarcoma and triple negative breast cancer models. Wnt-signaling was assessed in endothelial and tumor cells treated with SFRP2 mAb using Western blotting. Pharmacokinetic (PK) and biodistribution data were generated in tumor-bearing and non-tumor bearing mice. SFRP2 mAb was shown to induce anti-tumor and anti-angiogenic effects in vitro, and inhibit activation of ß-catenin and NFATc3 in endothelial and tumor cells. Treatment of SVR angiosarcoma allografts in nude mice with the SFRP2 mAb decreased tumor volume by 58% compared to control (p=0.004). Treatment of MDA-MB-231 breast carcinoma xenografts with SFRP2 mAb decreased tumor volume by 52% (p=0.03) compared to control, while bevacizumab did not significantly reduce tumor volume. Pharmacokinetic studies show the antibody is long circulating in the blood and preferentially accumulates in SFRP2-positive tumors. In conclusion, antagonizing SFRP2 inhibits activation of ß-catenin and NFATc3 in endothelial and tumor cells, and is a novel therapeutic approach to inhibiting angiosarcoma and triple negative breast cancer.
Keywords: angiosarcoma, breast carcinoma, endothelial cells, NFAT, angiogenesis
INTRODUCTION
Angiogenesis is the growth of new capillary blood vessels, and is a critical component of solid tumor growth(1). Limiting angiogenesis with bevacizumab (a monoclonal antibody to vascular endothelial growth factor (VEGF)) has resulted in an increase in overall survival in patients with metastatic colon(2), lung(3), renal cell carcinoma(4), and glioblastoma(5), and in progression free survival in patients with metastatic breast cancer(6). An important problem in the field of angiogenesis is that not all patients’ tumors respond to anti-VEGF therapy, and of those that respond, most eventually progress. There are several proposed mechanisms for anti-VEGF resistance(7), one of which may be tumor heterogeneity of angiogenic factors, such that other important angiogenesis stimulators are activating angiogenesis even while VEGF is inhibited. There is a critical need for the discovery of novel angiogenesis factors with the subsequent development of targeted angiogenesis inhibitors that are effective in tumors non-responsive to anti-VEGF therapy.
Our laboratory has recently discovered that secreted frizzled related protein 2 (SFRP2) is a novel angiogenesis stimulator in vitro and in vivo(8). While conducting genomic profiling of breast tumor vascular cells obtained by laser capture microdissection, we identified SFRP2 as a gene with 6-fold increased expression in breast tumor endothelium as compared to normal vessels (9). We found using immunohistochemistry that SFRP2 is present in the vasculature of triple negative, Her2/neu positive, and estrogen receptor positive breast tumors(8), as well as a wide variety of human tumors including angiosarcoma.
The angiogenic activity of SFRP2 is mediated by activating the non-canonical Wnt calcineurin/ nuclear factor of activated T-cells c3(NFATc3) pathway(10). NFAT is a transcription factor that plays a critical role in mediating angiogenic responses, including VEGF induced angiogenesis(11, 12). Based on the angiogenic activity of SFRP2, its expression in tumor vasculature, and its activation of NFATc3, we hypothesized that SFRP2 is a therapeutic target for inhibiting angiogenesis and tumor growth. However, there is a discrepancy in the literature as to whether SFRP2 is an antagonist of the Wnt/ ß-catenin pathway(13) (suggesting a role in tumor suppression) or agonist of ß-catenin(14-18) (suggesting a role in tumor promotion). This raises questions as to whether antagonizing SFRP2 could potentiate ß-catenin signaling in tumor cells causing an increase in tumor growth.
To more clearly define the role that SFRP2 plays in tumor growth we generated a monoclonal antibody to SFRP2. This enabled us to elucidate the effects of SFRP2 antagonism on tumor growth in vivo and the canonical and non-canonical Wnt signaling pathways in endothelial cells and tumors cells in vitro. Given that angiosarcoma is a lethal disease with very limited therapeutic options, and triple negative breast cancer has limited therapeutic options beyond chemotherapy, we focused our studies on these two aggressive tumor types for which there is a desperate need for novel therapies. The major findings of our study are that SFRP2 antagonism results in inhibition of ß-catenin and NFATc3 activation in both endothelial cells and tumor cells. Furthermore, SFRP2 mAb can inhibit the growth of both angiosarcoma and triple negative breast carcinoma in vivo. These studies clarify the role of SFRP2 in the Wnt-signaling pathway and validate SFRP2 as a promising new target for angiosarcoma and triple negative breast cancer.
MATERIALS AND METHODS
Cell culture
2H11 mouse endothelial cells (ATCC®) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose (Sigma-Aldrich, St. Louis, MO) with 10% FBS and 1% penicillin/streptomycin (v/v). MDA-MB-231 cells (ATCC®) were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin. ATCC provides authenticated cell line identity, and 2H11 and MDA-MB-231 cells were used within 6 month of purchase. SVR angiosarcoma cells were obtained from American Type Culture Collection (ATCC®, Manassas, VA) and cultured in low-glucose DMEM with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO). In addition, SVR angiosarcoma cells were tested negative by Research Analytic Diagnostic Laboratory (Columbia, MO) for PCR evaluation for: Ectromelia, EDIM, LCMV, LDEV, MHV, MNV, MPV, MVM, Mycoplasma sp., Polyoma, PVM, REO3, Sendai, TMEV GDVII. All cells were cultured at 37°C in a humidified 5% CO2-95% room air atmosphere.
Antibodies and proteins
The following antibodies were purchased from Santa Cruz Biotechnology, Inc: ß-catenin (sc-59893); and human SFRP2 recombinant protein. Recombinant mouse SFRP2 protein was purchased from R&D Systems, Inc., (Minneapolis, MN). The nuclear loading control of TATA binding protein TBP antibody (ab63766), NFAT4 (which is NFATc3) (ab96328) and Ki-67 were purchased from Abcam, Inc. (Cambridge, MA). CD31 primary antibody was purchased from NeoMarkers (Fremont, CA). Secondary antibodies ECL anti-mouse IgG, HRP-linked whole antibody (NA931) and ECL anti-rabbit IgG, HRP-linked whole antibody (NA934) were purchased from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ).
SFRP2 monoclonal antibody production and purification
Peptides to 5 epitopes from SFRP2 were synthesized, and mice were immunized against one of the 5 peptide sequences. Peptide sequences were designated peptide A-E (Peptide A: EACKNKNDDDNDIMETLC; Peptide B: EITYINRDTKIILETKSKTC; Peptide C: ITSVKRWQKGQREFKRISRSIRKLQC; Peptide D: GQPDFSYRSNC; Peptide E: DMLECDRFPQDNDLC). Mice were immunized twice on three week intervals with 50μg of antigen in 100μL Gerbu Adjuvuant via the intraperitoneal route. An enzyme-linked immunosorbent assay (ELISA) was performed to determine the titer of the mice to the peptides. Functional activity of the SFRP2 antibodies was evaluated by their ability to inhibit SVR angiosarcoma tube formation in vitro. Based on functional activity, “peptide B” was selected for the production of a monoclonal antibody. A tertiary injection of antigen was performed and the mice were boosted by a single intraperitoneal injection with 50μg of antigen in 100μL Gerbu Adjuvuant (GERBU Biotechnik GmbH, Germany). The titers to the immunogen in the injected mice were elevated, and two mice were selected for spleen harvest. The mice selected underwent a final intraperitoneal immunization three weeks after the last immunization. These mice were sacrificed 3 days after the final boost and blood was collected. The spleen was removed and fused with myeloma cells for hybridoma formation. The fusion of the spleen cells was with P3X63-Ag8.653 (ATCC CRL-1580, Manassas, VA) cells using a 50% polyethyleneglycol solution (Sigma-Aldrich, St. Louis, MO). The fusion was plated in 96 well plates at a total cell concentration of ~1.5 × 105 cells per well in the HAT selection media. The fusion plates were fed after 7 days with HAT selection media (hypoxanthine-aminopterin-thymidine) (Sigma-Aldrich, St. Louis, MO), which consisted of 10% FBS, 1x Glutamax (Life Technologies, Carlsbad, CA), 1x Penn/Strep, DMEM base (high glucose), and 1x HAT fusion plates were screened 14 days after the fusion was performed. Screening was performed with ELISA for “peptide B” and mouse recombinant SFRP2 and for functional activity by evaluating inhibition of SVR angiosarcoma cell tube formation in a Matrigel tube formation assay (see tube formation methods). Clones with the best functional activity at inhibiting tube formation in vitro were selected for further subcloning, and subclone 80.8.6 had the highest functional activity in vitro. The isotype of the SFRP2 MAb 80.8.6 was determined by the Isostrip Mouse Monoclonal Isotyping Kit (Roche Applied Science, Indianapolis, IN).
The antibody was purified through a HiTrap Protein G HP column (GE Healthcare, Uppsala, Sweden) and Detoxi-Gel Endotoxin Removing Column (Pierce/Thermo Scientific, Rockford, IL). The antibody was solubilized in buffer 20 mM Sodium Phosphate, 100 mM NaCl pH 5.5. A negative control IgG2ak subclone 29 that had no functional activity in inhibiting angiosarcoma tube formation in vitro was purified in a similar fashion for use as a negative control for in vivo assays.
Angiosarcoma and endothelial tube formation assay
ECMatrix (Millipore Corp, Billerica, MA) was thawed, diluted, and solidified into wells of a 96-well plate according to the manufacturer’s instructions. SVR angiosarcoma cells were serum starved (2% FBS) overnight and seeded onto the matrix at a concentration of 1 × 104 per well in 150μL DMEM with 10% FBS. To screen hybridomas for functional activity, supernatants with hybridoma (undiluted, 1:5, and 1:10), or media alone control, was added to the wells. For testing efficacy of purified 80.8.6 SFRP2 MAb, a 0.5μg/mL to 500 μg/mL dose curve was added to the wells and the plates were returned to 37°C, 5% CO2 for 6-8 hours, and isotype matched IgG2ακ (Biolegend, San Diego, CA) 100 μg/ml was used for control. 2H11 endothelial cells were serum starved in DMEM with 2% FBS overnight, and then seeded onto the matrix at 12,500 cells/well in 150 μl of DMEM with 3% FBS and supplements. Control cells received buffer alone or control IgG2ακ 50 μg/ml; SFRP2-treated cells received mouse recombinant SFRP2 7nM; and SFRP2 MAb 80.8.6 treated cells received mouse recombinant SFRP2 7nM with SFRP2 MAb (0.5 μg/ml, 5 μg/ml, or 50 μg/ml). The plates were returned to 37°C, 5% CO2 for 6 hours. Images were acquired using the Nikon Eclipse TS100 microscope at x4 magnification with a Nikon CoolPix 995 digital camera. Results were quantified by counting the number of branch points.
Proliferation assays
SVR angiosarcoma cells and MDA-MB-231 cells were plated in 24 well plates at a concentration of 20,000 cells per well in DMEM with 2%FBS and allowed to attach overnight incubated at 37°C. Media was exchanged for DMEM with 5% FBS, and the cells were treated with SFRP2 MAb at 100 μg/mL or IgG2ακ 100 μg/ml. At 24 hours of incubation the cells were trypsinized (trypsin, Gibco, Grand Island, NY) and resuspended in media containing serum. Cells were counted using the TC10 Automated Cell Counter (Bio-Rad, Hercules, CA).
Scratch wound migration assays
MDA-MB-231 cells were seeded at a concentration of 9,000 cells/well in a 96 well plate in DMEM with 10%FBS. After 24 hours, cells were starved in DMEM with 1% FBS overnight and then a scratch wound was made using a 20 μl pipette tip and the media was changed to DMEM with 5% FBS. Cells were treated with IgG2ακ 100 μg/ml, or SFRP2 MAb 100 μg/ml, in quadruplicate. The distance of the wound was measured with an ocular micrometer from 0 to 36 hours. Percent wound closure was measured with the formula [(Ti - Tx) / Ti] X 100; where Ti = initial wound distance and Tx= subsequent wound distance measurements.
Western blot analyses for NFATc3 and β-catenin in SFRP2 MAb treated cells
2H11 mouse endothelial cells, SVR angiosarcoma cells, and MDA-MB-231 cells were grown to 80-90% confluence in DMEM with 10% FBS. The cells were serum starved in DMEM with 2% FBS overnight. The following day the media was changed to DMEM with 2% FBS. Control cells received equal volumes of buffer; SFRP2-treated cells received mouse recombinant SFRP2 (7nM); and SFRP2 mAb treated cells received SFRP2 mAb (100 μg/ml) with mouse recombinant SFRP2 (7nM), and cells were incubated for one hour. Nuclear proteins were extracted by using NE-PER nuclear and cytoplasmic extraction reagent (Thermo Scientific, Rockford, IL). Western blot was performed as described(10). Dosimetry was obtained and normalized to TATA levels.
Tumor studies with angiosarcoma allografts in vivo
Mouse studies were approved by the Institutional Animal Care and Use Committee at the University of North Carolina. SVR cells (1 × 106) were injected into the subcutaneous dorsum of 6 week old nude female mice obtained from Charles River (Wilmington, MA). Because this is a very aggressive, fast growing tumor cell line, treatment was started the day following inoculation. To determine the multi-dose maximal tolerated dose, mice (n=15 per group) received purified SFRP2 mAb (2 mg/kg, 4 mg/kg, or 20 mg/kg) i.v. or antibody buffer control via tail injections and were treated twice weekly for 4 doses. We selected the dose range to test for the SFRP2 mAb based on the dose range of bevacizumab in mice(19). We did this because both antibodies have an IgG backbone and are binding to secreted angiogenic factors. Serial caliper measurements of perpendicular diameters were used to calculate tumor volume using the following formula: [(L (mm) x W (mm) x H (mm)) X0.5 × 1000] and the ratio of treated to control tumor volume (T/C) was determined for the last time point. Mice were monitored daily for body conditioning score and weight. Mice were sacrificed when the controls reached 1.4 cm diameter, and tumors were resected and placed in formalin. To rule out an effect of isotype matched control on tumor growth, mice were treated in the same fashion comparing buffer control to IgG isotype matched negative control clone 29 (4 mg/kg i.v. twice weekly).
Tumor studies with triple negative breast cancer xenografts in vivo
MDA-MB-231 xenografts were established in 5- to 6-week-old nude mice. Mice were inoculated with 1 × 106 cells s.c., and the animals were randomly allocated to control (n=11), SFRP2 mAb 4 mg/kg iv twice weekly (n=12); bevacizumab (Genentech, San Francisco, CA) 5 mg/kg iv twice weekly (n=12); or SFRP2 mAb 4 mg/kg iv twice weekly + bevacizumab 5 mg/kg iv twice weekly (n=12) for 30 days. Treatment began on day 14 after tumor inoculation when average tumor size was approximately 100 mm3. Tumors were harvested when tumor diameter reached 2 cm. To rule out an effect of isotype matched control on tumor growth, mice were treated in the same fashion comparing buffer control to IgG isotype matched negative control clone 29 (4 mg/kg i.v. twice weekly).
Immunohistochemistry and apoptosis
Tumors were fixed in paraffin, sectioned, and immunohistochemistry was performed as previously described (10) using Ki-67 (1:100 dilution) as the primary antibody. Tumor proliferation was quantified as the number of positively staining cells/unit area (x400), using the mean of 3 fields. To assess microvascular density, immunohistochemistry was performed as described (10) using CD31 (1:200 dilution) and adding an antigen retrieval step using 2N HCL for 10 minutes. Slides were evaluated for the presence of CD31 staining in tumor endothelium. Tumor vascularity was quantified as described previously to identify the number of microvessels/unit area (x400)(20-23). The mean of three fields judged to have the greatest numbers of microvessels was used for comparison between control and SFRP2 MAb treated tumors. To determine apoptosis, tumors were sectioned and processed as previously described(20) using the ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA). Tumor apoptosis was quantified as the number of positively staining nuclei /unit area (x400), using the mean of 5 fields.
PK radiolabeling of SFRP2 monoclonal antibody
Iodination of SFRP2 mAb was performed using the IODO-BEADS method as described(24) . Briefly, 300 μg of SFRP2 mAb in 130 μl of PBS was incubated for 25 min with 3 mCi of Na-125I and three IODO-BEADS (Pierce, Rockford, IL). The reaction mixture was separated from the IODO-BEADS by filtration (Centricon-10 from Millipore, Billerica, MA). The labeled 125I-SFRP2 mAb was mixed with unlabeled SFRP2 mAb to yield three different specific activities depending on the intended dose of SFRP2 mAb. The target counts per minute (cpm) injected for all mice was 5,000,000 cpm per 250 μl injection. The specific activities were 3.31-3.99 × 105 cpm/μg SFRP2 mAb, 3.40-4.09 × 104 cpm/μg SFRP2 mAb, and 1.06-1.28 × 104 cpm/μg SFRP2 mAb for the 0.4 mg/kg, 4.0 mg/kg, and 10 mg/kg doses, respectively.
Single dose pharmacokinetic studies in mice
125 I-SFRP2 mAb was administered to nude mice i.v. via tail-vein injection at doses of 0.4 mg/kg, 4 mg/kg, or 10 mg/kg. Blood samples were collected in serum separator tubes (BD, Franklin Lakes, NJ), allowed to clot at room temperature for 1 hr, and processed by centrifugation (11,000 rpm for 10 min) to obtain serum. Time points were pre-test and 5 min, 1 day, 2 days, 7 days, 14 days and 21 days using 3-5 mice per time point. For tumor-bearing mice, day 21 samples were not collected since the mice were sacrificed after day 14 due to tumors reaching the maximum diameter of 2 cm. For tissue distribution studies, tissue samples were collected at 1, 48, 168, 336, and 840 hr. Collection of tissues (tumor, spleen, lungs, heart, liver, kidney, skeletal muscle, stomach, small intestines, large intestines, lymph nodes, skin, and fat) was performed immediately after the blood was collected.
Quantitation of SFRP2 mAb concentration in serum and tissue
The radiolabeled SFRP2 mAb in serum was determined by gamma counting of trichloroacetic acid (TCA) precipitable radioactivity. After counting the total radioactivity in 50 μl of serum (in duplicates), an equal volume of 20% TCA was added to each aliquot, samples were centrifuged at 12,000 rpm for 10 min, and TCA-soluble radioactivity in the supernatant was determined. An aliquot of 50 μl of the supernatant was counted for soluble 125I cpm. The fraction of free iodine was calculated using the formula: [2 × average soluble cpm/average total cpm x 100%]. In all studies, the % free iodine was <3%. All serum and tissue concentrations of 125I-SFRP2 mAb were decay corrected to the time of injection. Blood-to-tissue (B/T) ratios for a given tissue at a given time point were calculated using the ratio of % injected dose of the SFPR2 mAb in the blood/% injected dose of the SFRP2 mAb in the given tissue.
Pharmacokinetic calculations
Pharmacokinetic parameters were determined based on the mean concentration values for 3-5 animals per time point. A non-compartmental analysis module (Model 201) of the pharmacokinetic software package WinNonlin, version 5.1 (Pharsight, Carey, NC) was used. The area under the blood concentration-versus-time curve (AUC) was calculated using the linear trapezoidal method. The slope of the apparent terminal half-life (T1/2) was estimated by log-linear regression using at least three data points and the terminal rate constant (λ) was derived from the slope. AUC0-∞ was estimated as the sum of the AUCo-t (where t is the time of the last measurable concentration) and Ct /λ. The apparent terminal half-life (T1/2) was calculated as 0.693/λ. For purposes of determining blood concentration of SFRP2 MAb, the volume of serum and blood were assumed to be equivalent.
Statistics
Results from in vivo and in vitro experiments are expressed as means ± standard error. Statistical comparisons between groups are made with a two-tailed T-Test. Statistical significance was accepted at P ≤ 0.05. Statistical analyses were performed using the GraphPad Prism Software version 4.0 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Generation of monoclonal antibody to SFRP2
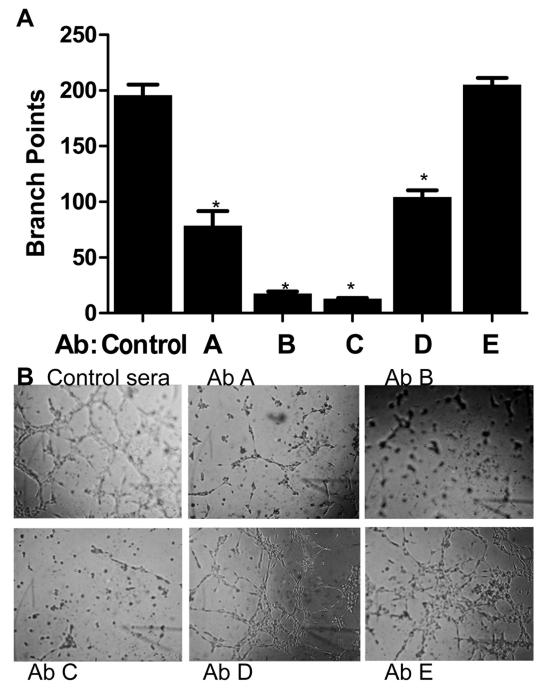
To generate a functionally active SFRP2 monoclonal antibody, we screened subclones for binding to SFRP2 by ELISA, and for the ability to inhibit SVR angiosarcoma tube formation in vitro. This assay was chosen because SVR cells produce SFRP2 protein(8) (Supplemental Fig. 1), and silencing SFRP2 inhibited angiosarcoma tube formation(8) in a Matrigel tube formation assay. Sera from the mouse immunized against the epitope corresponding to amino acids which we have “peptide B” and “peptide C” had a 10-fold reduction in SVR angiosarcoma tube formation compared to control mouse sera (p<0.001), indicating that antibodies to these peptide sequences are the most functionally active (Fig.1). The epitopes for “peptides B and C” are 100% homologous between mouse and human SFRP2. The antibody raised against “peptide B” was chosen for generation of a monoclonal antibody. Subclones of hybridomas were screened by ELISA for “peptide B” (Supplemental Fig. 2, which shows strong reaction of subclone 80.8.6), and subclones with strong reactivity to “peptide B” were further selected based on potency at inhibiting angiosarcoma tube formation in vitro. A final selection was made by assessing activity in an SVR angiosarcoma scratch wound migration assay (Fig. 2C), which demonstrates the SFRP2 mAb inhibits angiosarcoma migration (p=0.03). Isotyping revealed that SFRP2 mAb 80.8.6 is an IgG2a, heavy chain kappa light antibody, and Western blot shows this antibody binds to both mouse and human recombinant SFRP2 (Supplemental Fig. 3). The overall isoelectric point of the protein as determined by isoelectric focusing gel electrophoresis is 8.5.
Figure 1.
Polyclonal antibodies to different epitopes of SFRP2 inhibit SVR angiosarcoma tube formation.
ECMatrix was thawed, diluted and solidified in a 96 well plate. SVR angiosarcoma cells were seeded onto the matrix at a concentration of 1×104 cells / well in 150 μl of DMEM with 10% FBS. Sera from mice immunized against peptides sequences from SFRP2 (see Materials and Methods for peptide sequences, designated AbA, AbB, AbC, AbD, AbE, or control sera), were added to the wells at 1:100 dilution and incubated for 8 hours. Results were quantified by counting the number of branch points. SVR cells in control sera formed tubes, whereas antibodies to peptide A, B, C, and D all inhibited tube formation. AbB and AbC had the greatest inhibition (p<0.001, n=4 for all groups). B) Representative photographs of angiosarcoma tube formation.
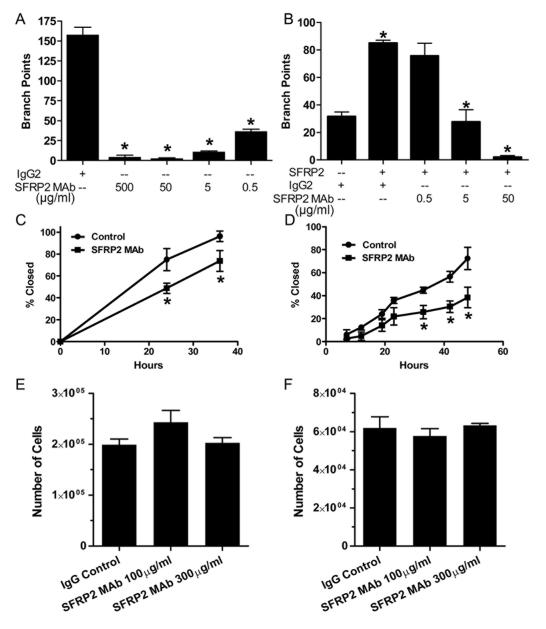
Figure 2.
SFRP2 mAb inhibits tube formation and migration in vitro, with no effect on proliferation.
A) SVR angiosarcoma cells were seeded onto the matrix at a concentration of 1×104 cells / well in 150 μl of DMEM with 10% FBS. A dose curve of SFRP2 mAb, or an IgG2ακ (500 μg/ml) was added to the wells and the plates were incubated for 8 hours. Results were quantified by counting the number of branch points. Isotype matched control SVR cells formed tubes, whereas the SFRP2 mAb significantly inhibits angiosarcoma tube formation (p<001). B) SFRP2 MAb competition binding assay in 2H11 endothelial cell tube formation assay. 2H11 mouse endothelial cells were plated in Matrigel with IgG2ακ control (50 μg/ml), or with mouse recombinant SFRP2 (7nM) plus IgG2ακ (50 μg/ml), or with SFRP2 (7nM) plus increasing concentrations of SFRP2 mAb 80.8.6. After 6 hours the number of branch points were counted. SFRP2 stimulated tube formation compared to IgG control (p=0.001). Control IgG did not neutralize the effect of SFRP2, however SFRP2 mAb 80.8.6 neutralized the effect of SFRP2 stimulated tube formation (p≤ 0.003). C) SVR Angiosarcoma Scratch Wound Migration Assay. Angiosarcoma cells were plated at 10,000 cells/ well and allowed to attach overnight. A scratch was formed with a pipette tip and media was changed to Hy selection media (1:5 dilution) or supernatants from hybridomas secreting SFRP2 mAb (1:5). The percent wound closed was monitored over 40 hours. SFRP2 mAb inhibited wound closure by 47% compared to IgG control (*p≤0.04). D) MDA-MB-231 Scratch Wound Migration Assay. Breast cancer cells were plated at 10,000 cells/ well and allowed to attach overnight. A scratch was formed with a pipette tip and media was changed to IgG control 100ug/ml or SFRP2 mAb 100ug/ml. The percent wound closed was monitored over 48 hours. SFRP2 mAb inhibited wound closure by 47% compared to IgG control (*p≤0.04). E) SVR Angiosarcoma or F) MDA-MB-231 Proliferation Assay. Cells were plated and allowed to attach overnight. Media was changed and cells were incubated for 24h with IgG 100 μg/ml control, of SFRP2 mAb 100 or 300 μg/ml. There was no effect of the SFRP2 MAb on angiosarcoma (D) or breast cancer (E) cell number compared to control.
Effect of SFRP2 antagonism endothelial and tumor cell tube physiology in vitro
To evaluate the dose range for which the purified SFRP2 mAb would inhibit tube formation, SVR angiosarcoma cells were plated in a Matrigel tube assay with or without SFRP2 MAb 80.8.6. Tube formation was completely inhibited at SFRP2 mAb concentrations of 5-500 μg/ml, and was inhibited by 75% at 0.5ug/ml (Fig. 2A) compared to control. This is effect was not due to toxicity, as there was no difference in cell number after 24 hours incubation with SFRP2 mAb (100 and 300 μg/ml) in either angiosarcoma cells (Fig. 2E) or MDA-MB-231 breast cancer cell (Fig. 2F) in vitro. To further demonstrate that the SFRP2 mAb 80.8.6 was functionally active at inhibiting SFRP2 angiogenic function, we performed an in vitro competition binding assay in 2H11 mouse endothelial cells. When 2H11 endothelial cells were treated with mouse recombinant SFRP2 there was a 2.3-fold increase in tube formation (p=0.001, Fig. 2B). When SFRP2 mAb was incubated with SFRP2, tube formation was not induced, indicating that the SFRP2 mAb 80.8.6 antibody is functionally active at neutralizing SFRP2 (Fig. 2B).
Some angiogenesis inhibitors, such as endostatin(25), inhibit only endothelial cells with no effect on tumor cells; while others, such as 2-methoxyestradiol(26), inhibit both the endothelial and tumor cell compartments. To evaluate whether SFRP2 antagonism would inhibit tumor cells directly, we studied effects of SFRP2 mAb on MDA-MB-231 breast cancer cell migration using a scratch wound migration assay. MDA-MB-231 cells produce endogenous SFRP2 protein as demonstrated by Western blot of whole cell lysates (Supplemental Fig. 1). SFRP2 mAb 80.8.6 100 μg/ml inhibited MDA-MB-231 cell migration by 47% compared to IgG2ak 100 μg/ml (p=0.04) (Fig. 2D). This demonstrates that the SFRP2 mAb inhibits both the endothelial and tumor cell compartments.
Effect of SFRP2 antagonism on Wnt signaling in endothelial cells
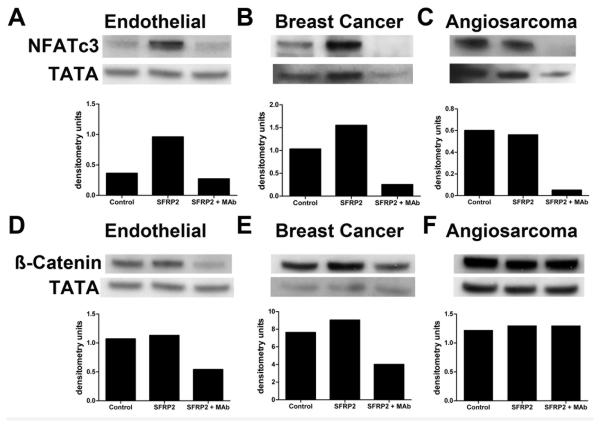
There is a discrepancy in the literature as to whether SFRP2 is an antagonist of the Wnt/ ß-catenin pathway(13) or agonist of ß-catenin(14-18). When ß-catenin or NFAT are activated they translocate into the nucleus, resulting in increased nuclear accumulation. To define the effects of SFRP2 stimulation and SFRP2 antagonism on the Wnt/ β-catenin and calcineurin/NFATc3 pathways, we treated endothelial, angiosarcoma, and breast cancer cells with control, recombinant SFRP2, or SFRP2 mAb. Nuclear proteins were extracted, and Western blot was performed probing for ß-catenin and NFATc3. For NFATc3, there was an increase in nuclear accumulation compared to control after treatment with recombinant SFRP2, and a decrease in nuclear accumulation after treatment with the SFRP2 mAb in 2H11 endothelial (Fig. 3A), MDA-MB-231 breast cancer (Fig. 3B), and SVR angiosarcoma cells (Fig. 3C). Nuclear β-catenin was elevated in control 2H11 endothelial cells and MDA-MB-231 breast cancer cells, and this was not decreases with treatment with SFRP2 (in fact there was a small increase in SFRP2 treated cells compared to control); however the SFRP2 mAb decreased nuclear β-catenin in both cell types (Fig. 3D, E). In angiosarcoma cells, there was a high level of ß-catenin in control cells, which was not altered with recombinant SFRP2 protein nor with the SFRP2 mAb. (Fig. 3F). In summary, SFRP2 mAb inhibits the non-canonical calcineurin/NFAT pathway in endothelial, angiosarcoma, and MDA-MB-231 cells; and the canonical Wnt-β-catenin pathway in endothelial and breast cancer cells, but does not play a role in angiosarcoma. Taken together this suggests that SFRP2 antagonism should inhibit, not promote, tumor growth.
Figure 3.
Effect of SFRP2 and SFRP2 mAb on Wnt signaling in endothelial cells.
2H11 endothelial cells, MDA-MB-231 breast cancer cells, and angiosarcoma cells were treated with control, mouse recombinant SFRP2 (7nM), or SFRP2 (7nM) + SFRP2 mAb 100 μg/ml) for 1 hour. Cells were lysed and nuclear fraction was collected and subjected to Western blotting with antibodies to ß-catenin or NFATc3, and TATA (nuclear loading control. Dosimetry readings were normalized to TATA levels. Treatment with SFRP2 increased nuclear NFATc3 compared to control in endothelial cells(A), MDA-MB-231 cells (B) and angiosarcoma cells (C); which was inhibited with the SFRP2 mAb. Nuclear β-catenin was elevated in control 2H11 endothelial cells (D) and MDA-MB-231 breast cancer cells (E), and this was not decreased with treatment with SFRP2; rather the SFRP2 mAb decreased nuclear β-catenin in both cell types (D, E). In SVR angiosarcoma cells, nuclear β-catenin was elevated in control cells and was not altered with SFRP2 recombinant protein (F) nor the SFRP2 mAb. Full length gels are in Supplemental Fig. 4.
SFRP2 MAb inhibits the growth of primary tumors
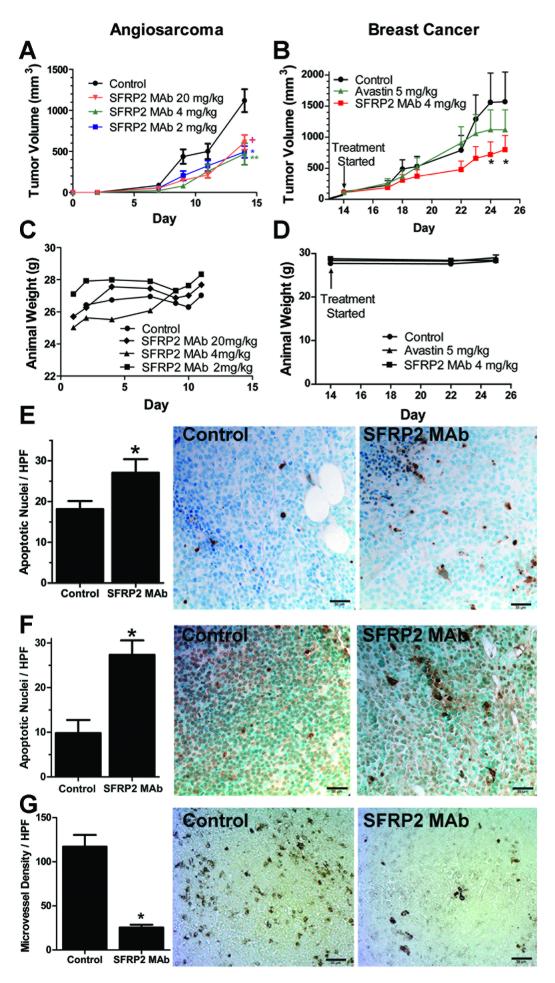
To determine the multi-dose maximum tolerated dose (MTD) of SFRP2 mAb, SVR angiosarcoma cells (1 × 106) were injected s.c. in 6 week old female nude mice. Treatment was initiated the day after inoculation so that the multi-dose MTD could be determined in this rapidly growing cell line. Mice (n=15 per group) received SFRP2 mAb (2, 4, or 20 mg/kg) or buffer control intravenously via tail-vein injections twice weekly for 4 doses. Mice treated with SFRP2 mAb 2 mg/kg had a 56% inhibition of tumor growth compared to control (p=0.001); SFRP2 mAb 4 mg/kg had a 58% inhibition (p=0.004); SFRP2 mAb 20 mg/kg had a 46% inhibition (p=0.01) (Fig. 4A). After 4 injections there was no weight loss or lethargy in the treated mice at all dosages, including the highest dose of 20 mg/kg (Fig. 4C). To rule out an effect of the IgG antibody on tumor growth, an additional experiment compared buffer control (n=8) to isotype matched IgG control (n=7) at 4 mg/kg twice weekly. There was no decrease in tumor volume with the IgG control (592 mm3 ± 169) compared to buffer control (515 mm3 ± 262), p=NS after 14 days of treatment.
Figure 4.
SFRP2 mAb inhibits angiosarcoma and breast cancer growth in vivo.
A) SVR angiosarcoma cells were injected (1× 106 cells s.c.) into mice, and the day after injection treatment was started with control buffer or SFRP2 mAb (n=15 per group). Mice treated with SFRP2 mAb 2 mg/kg had a 56% inhibition of tumor growth compared to control (**p=0.001); SFRP2 mAb 4 mg/kg had a 58% inhibition (*p=0.004); SFRP2 mAb 20 mg/kg had a 46% inhibition (+p=0.01). B) MDA-MB-231 breast cancer cells (1× 106 cells s.c.) were injected and treatment was started when tumors were palpable at 14 days (n=12 per group). Mice received either control buffer, bevacizumab (5 mg/kg) i.v. twice weekly, or SFRP2 mAb (4 mg/kg) i.v. twice weekly. Bevacizumab decreased tumor volume by 32% compared to control on day 24, which was not statistically significantly (p=0.32). The SFRP2 mAb inhibited tumor volume by 54% on day 24 (*p=0.03). There was no weight loss in mice with angiosarcomas (C) or breast tumors (D) treated with the SFRP2 mAb. E) The number of apoptotic nuclei/400x HPF for control SVR angiosarcoma tumors was increased in tumors treated with the SFRP2 MAb compared to control (*p=0.05, n=3). F) The number of apoptotic nuclei/400x HPF for control MDA-MB-231 breast tumors was increased in tumors treated with the SFRP2 mAb compared to control (*p=0.02, n=3). G) Microvascular density per HPF was decreased in MDA-MB-231 breast tumors treated with SFRP2 mAb compared to control (*p<0.001, n=3)
To evaluate the efficacy of the SFRP2 mAb in a second tumor cell line, we used the MDA-MB-231 triple negative breast cancer human xenograft model, and compared efficacy to bevacizumab, a monoclonal antibody to VEGF (n=12 per group). Treatment of mice with bevacizumab at 5 mg/kg i.v. every 3 days resulted in a 32% decrease in tumor volume compared to control on day 24, which was not statistically significantly (p=0.32). In contrast, the SFRP2 mAb (4 mg/kg i.v. every 3 days) inhibited tumor volume by 54% on day 24 (p=0.03, Fig. 4B). There was no weight loss (Fig. 4D) or signs of toxicity in the SFRP2 mAb treated mice. To rule out an effect of the IgG antibody having an effect on tumor growth, an additional experiment comparing buffer control (n=9) to isotype matched IgG control (n=9) at 4 mg/kg twice weekly was performed. There was no decrease in tumor volume with the isotype matched negative control compared to buffer control, p=NS after 14 days of treatment.
The proliferative index of tumors in the SFRP2 mAb and buffer-treated mice was at the same high level in both groups (data not shown) while the apoptotic index increased after SFRP2 mAb therapy. The number of apoptotic nuclei/400x high-power field (HPF) for control SVR angiosarcoma tumors was 18 ± 3 (n=3); which was increased in tumors treated with the SFRP2 mAb (27 ± 4, n=3, p=0.05) (Fig. 4E). The number of apoptotic nuclei/400x HPF for control MDA-MB-231 breast tumors was 10 ± 3 (n=3); which was increased in tumors treated with the SFRP2 mAb (27 ± 4, n=3, p=0.02) (Fig. 4F). The microvessel density/ 400x HPF for control MDA-MB-231 tumors was 117 ± 13, which was decreased in tumors treated with the SFRP2 mAb (25 ± 3, p <0.0001) (Fig. 4G).
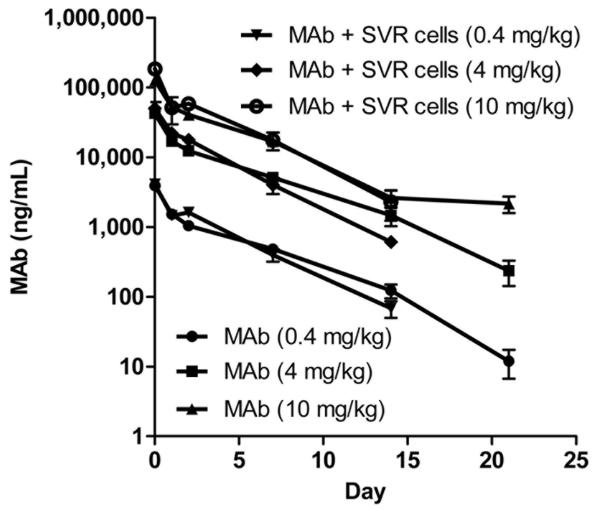
Pharmacokinetics and biodistribution of SFRP2 MAb
SFRP2 mAb was long circulating in the blood with an average T1/2 in the range of 53-89 hour (Fig. 5). In addition, the SFPR2 mAb was found to preferentially target the tumors versus all other organs except for the liver. For example, in tumor bearing mice, the blood/tissue ratio on day 14 was smallest in the liver (15:1) and tumor (16:1) as compared to all other organs (range: 39:1 to 255:1) proving that the tumor was a prime organ for accumulation of the SFRP2 mAb. As shown in Table 1, the SFPRP2 mAb in tumor bearing and non-tumor bearing mice exhibited dose-independent kinetics, as a one-way ANOVA analysis comparing T1/2 at different dose levels was not statistically significant (p=0.2847 and 0.1204, respectively). However, there was a statistically significant difference in T1/2 of the SFPR2 mAb in tumor-bearing and non-tumor bearing mice (p=0.0386).
Figure 5.
Single-dose pharmacokinetic profile of SFRP2 mAb in SVR-tumor bearing and non-tumor bearing mice.
SFRP2 mAb was long circulating in the blood with an average T1/2 in the range of 53-89 hr. 125I-labelled SFRP2 mAb was administered to mice at doses of 0.4, 4, and 10 mg/kg. At various time-points, SFRP2 mAb in serum was determined by gamma counting of tricholoacetic-acid (TCA) perceptible radioactivity. SFRP2 mAb in both tumor-bearing and non-tumor bearing mice exhibited dose-independent kinetics as a one-way ANOVA analysis comparing T1/2 at different dose levels was not statistically significant (p=0.2847 and 0.1204, respectively). However, there was a statistically significant difference in the T1/2 of the SFPR2 mAb in tumor-bearing and non-tumor bearing mice (p=0.0386).
Table 1.
Pharmacokinetic Parameters of SFRP2 mAb in Tumor Bearing and Nontumor Bearing Mice.
| SFRP2 mAb Dose (mg/kg) |
Tumor bearing? |
AUC∞ (ng/ml*h) | Cmax (ng/ml) | VD (ml) | T1/2 (h) |
|---|---|---|---|---|---|
| 10 | No | 8,265,587 ± 564,635 | 125,633 ± 13, 970 | 4.65 ± 1.00 | 89.33 ± 23.64 |
| 10 | Yes | 9,440,466 ± 2,801,212 | 185,760 ± 24, 794 | 3.20 ± 0.29 | 68.57 ± 15.66 |
| 4 | No | 2,610,899 ± 338,576 | 46,637 ± 4,903 | 5.10 ± 1.58 | 75.37 ± 20.10 |
| 4 | Yes | 2,781,180 ± 396,051 | 53,040 ± 10,028 | 3.36 ± 0.21 | 53.92 ± 7.81 |
| 0.4 | No | 229,544 ± 29,684 | 4,444 ± 0 | 4.58 ± 0.74 | 61.02 ± 14.87 |
| 0.4 | Yes | 250,358 ± 51,895 | 4,566 ± 178 | 4.20 ± 0.73 | 59.82 ± 10.85 |
DISCUSION
Secreted frizzled-related protein 2 (SFRP2) belongs to a family of secreted factors involved in embryonic development. Due to its homology to the extracellular portion of the Wnt receptor Frizzled, SFRP2 has been implicated in binding to Wnts, thereby blocking Wnt binding to Frizzled receptors, and resulting in inhibition of β-catenin activation(13). This, in combination with data showing that SFRP2 is hypermethylated in certain tumors(27-29), has led to an assumption that SFRP2 is a tumor suppressor. However, there is a discrepancy in which several studies have shown that SFRP2 is an agonist (rather than an antagonist) of β-catenin (14-18), suggesting the reverse: that SFRP2 may promote tumor growth. If SFRP2 were to directly antagonize Wnt/ β-catenin signaling in tumors, then overexpression of SFRP2 in tumors should result in a decrease in tumor growth. To the contrary, SFRP2 has been found to be produced by the majority of malignant glioma cell lines, and SFRP2 overexpressing intracranial glioma xenografts were significantly larger than xenografts consisting of control cells in nude mice(30). Additionally, transient transfection of SFRP2 in renal cell carcinoma has been shown to increase tumor growth in vivo(31) . Another pathway through which SFRP2 could promote tumor growth is via its angiogenic function. We previously found that SFRP2 is a novel stimulator of angiogenesis via activation of the calcineurin/ NFAT pathway, and is overexpressed in malignant vessels of a wide variety of human tumors(8, 10).
To clarify whether SFRP2 is a therapeutic target to inhibit tumor growth, we generated a monoclonal antibody to SFRP2 to elucidate the effects of SFRP2 antagonism on endothelial and tumor cell growth and Wnt signaling in vitro, and tumor growth in vivo. The epitope that this antibody was raised against is 100% homologous to mouse and human SFRP2, and SFRP2 mAb 80.8.6 binds to both mouse and human recombinant SFRP2 by Western blot. In vitro data demonstrates that SFRP2 mAb 80.8.6 inhibits endothelial and angiosarcoma tube formation; and angiosarcoma and breast cancer cell migration; with no effect on proliferation. We expected that antagonizing SFRP2 would not affect proliferation, as our previous study showed that recombinant SFRP2 had no effect on endothelial cell proliferation(32). Additionally, the SFRP2 mAb blocked the activation of NFATc3 in endothelial cells, angiosarcoma cells, and MDA-MB-231 breast cancer cells. In contrast to the proposal that SFRP2 is an inhibitor of ß-catenin(13), we found that SFRP2 did not decrease endogenous nuclear ß-catenin in all three cell types; to the contrary the SFRP2 mAb decreased nuclear ß-catenin in endothelial cells and breast cancer cell, with no effect in angiosarcoma cells. This data demonstrates that antagonism of SFRP2 directly inhibits endothelial cell and tumor cell canonical and non-canonical Wnt signaling and oncogenic functions in vitro.
An essential test of whether SFRP2 is a tumor suppressor or tumor promoter can be demonstrated by antagonizing SFRP2 in tumor models in vivo. In this study we found that the SFRP2 mAb reduces tumor growth in two aggressive tumor types in vivo, angiosarcoma and triple negative MDA-MB-231 breast carcinoma; whereas bevacizumab did not significantly inhibit MDA-MB-231 xenograft growth. Pharmacokinetic and biodistribution studies showed that the SFRP2 mAb is long circulating and accumulates in the tumor, and demonstrates dose-independent kinetics. There was no weight loss or signs of toxicity at the highest dose tested, which was 5-times the efficacious dose.
Triple negative breast cancer and angiosarcoma are both aggressive malignancies for which there is a strong need for novel therapies. Angiosarcoma is a biologically aggressive vascular malignancy with a high metastatic potential and subsequent mortality. The 5-year overall survival for all patients with angiosarcoma is 30%, with a median overall survival of 24 months(33). The finding that antagonizing SFRP2 reduces angiosarcoma growth provides a new avenue for developing targeted therapy for this highly lethal disease.
Although the growth inhibition induced by the SFRP2 mAb in the MDA-MB-231 and angiosarcoma cells in vivo; and inhibition of NFATc3 in vitro was significant, it was not 100%. The mechanism for this is unknown, however a possible explanation for this is that the MDA-MB-231(34) and angiosarcoma(35) cell lines have been reported to express VEGF, and VEGF is known to activate the NFAT pathway and tumor growth. Future studies looking at whether combination therapy of anti-SFRP2 and anti-VEGF therapy are additive is warranted. Additionally, there was decreased efficacy of the SFRP2 mAb at a high concentration (20 mg/kg) compared to 4 mg/kg, indicating a bell shaped dose response curve. Although we do not know the reason for this, bell-shaped dose response curves are a common feature of angiogenesis inhibitors(36), and described for monoclonal antibodies(37).
In summary, these studies demonstrate that SFRP2 antagonism inhibits tumor growth and provides evidence that SFRP2 is a tumor promoter rather than a tumor suppressor. Therefore antagonism of SFRP2 with a monoclonal antibody is a therapeutic strategy to inhibit tumor growth via antagonizing canonical and non-canonical Wnt-signaling.
Supplementary Material
Acknowledgments
GRANT SUPPORT This work was supported by National Institute of Health (P50-CA58223 and 1R01CA142657-01A1 to NK-D) (R01 HL61656 to CP), North Carolina TraCS Large Pilot Award, University Cancer Research Fund, Nancy DeMore Foundation to NK-D; North Carolina Kickstart Commercialization Collaboration Award to CP and NK-D.
Abbreviation list
- SFRP2
secreted frizzled related protein 2
- NFAT
nuclear factor of activated T-cells
- mAb
monoclonal antibody
- PK
pharmacokinetic
- VEGF
vascular endothelial growth factor
- DMEM
Dulbecco’s modified eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- T1/2
terminal half-life
- AUC
area under the blood concentration versus time curve
- CPM
counts per minute Total word count= 5277
- FBS
fetal bovine serum Total number figures = 5
- TCA
trichloroacetic acid Total number of tables = 1
Footnotes
Potential Conflict of Interest: Discovery of Novel Targets for Angiogenesis Inhibition, Provisional patent application no. 61/053,397. Inventors: Nancy Klauber-DeMore, MD, Cam Patterson, MD, and Bradley Bone. Dr. Klauber-DeMore is co-founder, shareholder, Chief Scientific Officer, and Board Member of Enci Therapeutics, Inc., and shareholder and scientific advisory board member of b3bio, Inc. Dr. Patterson is co-founder, shareholder, President, and Board Member of Enci Therapeutics, Inc., and shareholder and Board Member of b3bio, Inc.
References
- ( 1).Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- ( 2).Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- ( 3).Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- ( 4).Pal SK, Figlin RA. Bevacizumab for metastatic renal cell carcinoma: a monoclonal antibody in a sea of small molecules. Expert Opin Biol Ther. 2010;10:1517–20. doi: 10.1517/14712598.2010.522904. [DOI] [PubMed] [Google Scholar]
- ( 5).Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- ( 6).Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- ( 7).Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–5. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- ( 8).Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res. 2009;69:4621–8. doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 9).Bhati R, Patterson C, Livasy CA, Fan C, Ketelsen D, Hu Z, et al. Molecular characterization of human breast tumor vascular cells. Am J Pathol. 2008;172:1381–90. doi: 10.2353/ajpath.2008.070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 10).Siamakpour-Reihani S, Caster J, Bandhu ND, Courtwright A, Hilliard E, Usary J, et al. The Role of Calcineurin/NFAT in SFRP2 Induced Angiogenesis-A Rationale for Breast Cancer Treatment with the Calcineurin Inhibitor Tacrolimus. PLoS One. 2011;6:e20412. doi: 10.1371/journal.pone.0020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 11).Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, et al. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem. 2004;279:50537–54. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- ( 12).Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004;199:1513–22. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 13).Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- ( 14).Esteve P, Sandonis A, Ibanez C, Shimono A, Guerrero I, Bovolenta P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development. 2011;138:4179–84. doi: 10.1242/dev.065839. [DOI] [PubMed] [Google Scholar]
- ( 15).Gehmert S, Sadat S, Song YH, Yan Y, Alt E. The anti-apoptotic effect of IGF-1 on tissue resident stem cells is mediated via PI3-kinase dependent secreted frizzled related protein 2 ( Sfrp2) release. Biochem Biophys Res Commun. 2008;371:752–5. doi: 10.1016/j.bbrc.2008.04.151. [DOI] [PubMed] [Google Scholar]
- ( 16).Lee JL, Chang CJ, Wu SY, Sargan DR, Lin CT. Secreted frizzled-related protein 2 ( SFRP2) is highly expressed in canine mammary gland tumors but not in normal mammary glands. Breast Cancer Res Treat. 2004;84:139–49. doi: 10.1023/B:BREA.0000018412.83348.ff. [DOI] [PubMed] [Google Scholar]
- ( 17).Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, et al. SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci U S A. 1997;94:13636–41. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 18).Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 ( Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 19).Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- ( 20).Klauber-DeMore N, Van Zee KJ, Linkov I, Borgen PI, Gerald WL. Biological behavior of human breast cancer micrometastases. Clin Cancer Res. 2001;7:2434–9. [PubMed] [Google Scholar]
- ( 21).Klauber N, Parangi S, Flynn E, Hamel E, D’Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997;57:81–6. [PubMed] [Google Scholar]
- ( 22).Klauber N, Rohan RM, Flynn E, D’Amato RJ. Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med. 1997;3:443–6. doi: 10.1038/nm0497-443. [DOI] [PubMed] [Google Scholar]
- ( 23).Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- ( 24).Vugmeyster Y, Szklut P, Tchistiakova L, Abraham W, Kasaian M, Xu X. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of humanized monoclonal anti-IL-13 antibodies with different IL-13 neutralization mechanisms. Int Immunopharmacol. 2008;8:477–83. doi: 10.1016/j.intimp.2007.12.004. [DOI] [PubMed] [Google Scholar]
- ( 25).O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- ( 26).D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci U S A. 1994;91:3964–8. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 27).Kinoshita T, Nomoto S, Kodera Y, Koike M, Fujiwara M, Nakao A. Decreased expression and aberrant hypermethylation of the SFRP genes in human gastric cancer. Hepatogastroenterology. 2011;58:1051–6. [PubMed] [Google Scholar]
- ( 28).Veeck J, Noetzel E, Bektas N, Jost E, Hartmann A, Knuchel R, et al. Promoter hypermethylation of the SFRP2 gene is a high-frequent alteration and tumor-specific epigenetic marker in human breast cancer. Mol Cancer. 2008;7:83. doi: 10.1186/1476-4598-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 29).Tanaka J, Watanabe T, Kanazawa T, Tada T, Kazama Y, Tanaka T, et al. Silencing of secreted frizzled-related protein genes in MSI colorectal carcinogenesis. Hepatogastroenterology. 2008;55:1265–8. [PubMed] [Google Scholar]
- ( 30).Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, et al. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19:4210–20. doi: 10.1038/sj.onc.1203783. [DOI] [PubMed] [Google Scholar]
- ( 31).Yamamura S, Kawakami K, Hirata H, Ueno K, Saini S, Majid S, et al. Oncogenic functions of secreted Frizzled-related protein 2 in human renal cancer. Mol Cancer Ther. 2010;9:1680–7. doi: 10.1158/1535-7163.MCT-10-0012. [DOI] [PubMed] [Google Scholar]
- ( 32).Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, et al. Secreted Frizzle-Related Protein 2 Stimulates Angiogenesis via a Calcineurin/NFAT Signaling Pathway. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 33).Espat NJ, Lewis JJ, Woodruff JM, Antonescu C, Xia J, Leung D, et al. Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma. 2000;4:173–7. doi: 10.1155/2000/575781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 34).Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–9. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- ( 35).Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–6. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 36).Reynolds AR. Potential relevance of bell-shaped and u-shaped dose-responses for the therapeutic targeting of angiogenesis in cancer. Dose Response. 2009;8:253–84. doi: 10.2203/dose-response.09-049.Reynolds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ( 37).Elliott S, Lorenzini T, Yanagihara D, Chang D, Elliott G. Activation of the erythropoietin ( EPO) receptor by bivalent anti-EPO receptor antibodies. J Biol Chem. 1996;271:24691–7. doi: 10.1074/jbc.271.40.24691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.