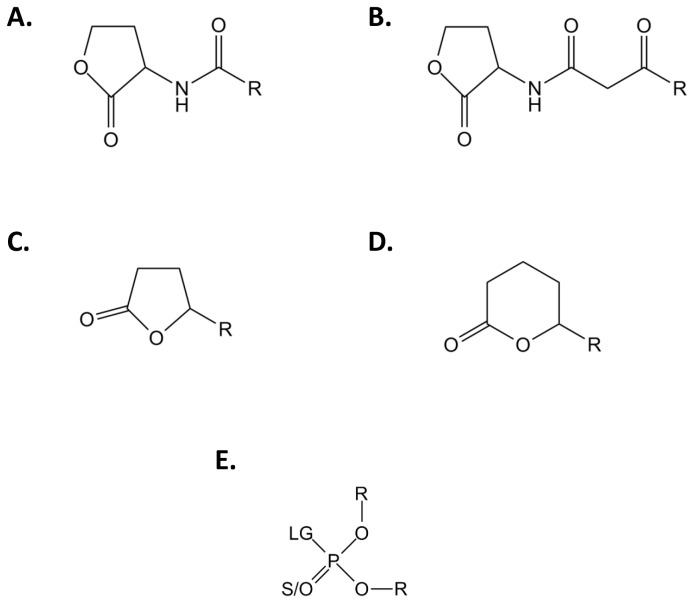
Figure 1. Generic chemical structure of SsoPox substrates.
Chemical structures of (A) Acyl-Homoserine Lactones, (B) 3-oxo-Acyl-Homoserine Lactones, (C) γ-lactones, (D) δ-lactones and (E) phosphotriesters are presented. For AHLs and γ/δ-lactones, R corresponds to the different size of the acyl chain. For phosphotriesters, R corresponds to the different nature of substituents; LG corresponds to the leaving group which can be F, S-R, O-R or CN. The terminal substituent could be a S atom if the molecule is a thiono-phosphotriester or an O atom if the molecule is an oxono-phosphotriester.