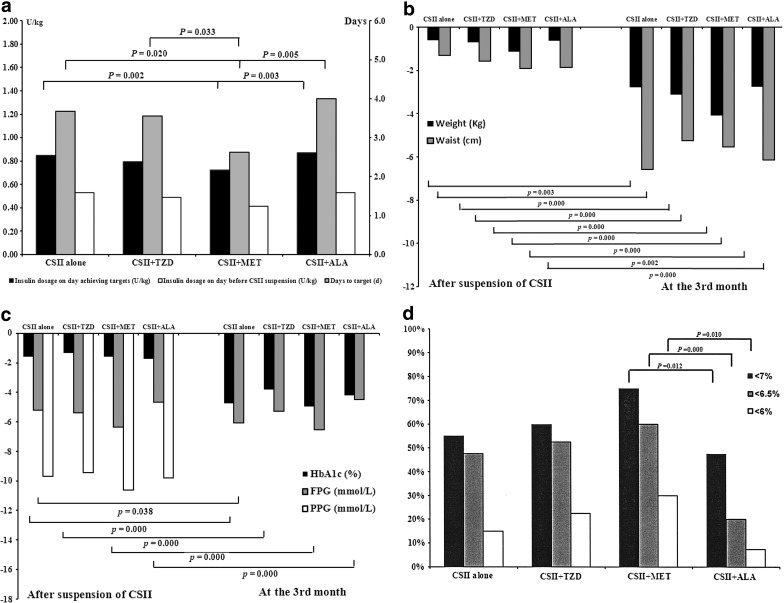
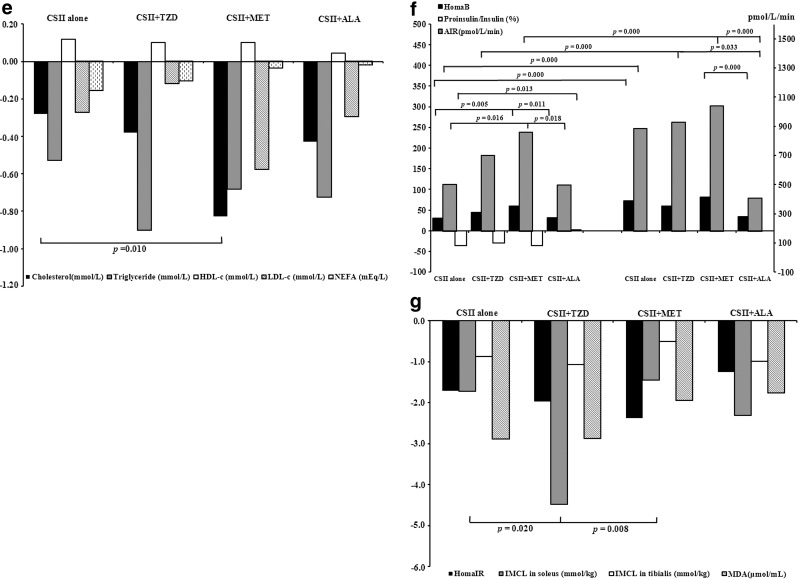
FIG. 2.
Comparisons of treatment efficacy in the four treatment groups. ALA, α-lipoic acid; MET, metformin; TZD, thiazolidinedione. (a) Insulin dosages and days to target during continuous subcutaneous insulin infusion (CSII) treatment period. (b) Changes from baseline after CSII suspension and at month 3 in body weight and waist circumference. (c) Changes from baseline after CSII suspension and at month 3 in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and postprandial plasma glucose (PPG). (d) Proportions of patients achieving HbA1c <7%, 6.5%, and 6% in each treatment group. (e) Changes from baseline after CSII suspension in lipid profile. HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NEFA, nonesterified fatty acid. (f) Changes from baseline after CSII suspension and at month 3 in homeostasis model assessment of β-cell function (HomaB), acute insulin response (AIR), and proinsulin to insulin ratio. (g) Changes from baseline after CSII suspension in homeostasis model assessment of insulin resistance (HomaIR) and intramyocellular lipid (IMCL) and malondialdehyde (MDA) levels.