Figure 2. Dynamic regulation of Beclin 1-VPS34 complex formation and autophagy by Bcl-2 family members and Beclin 1 dimerization.
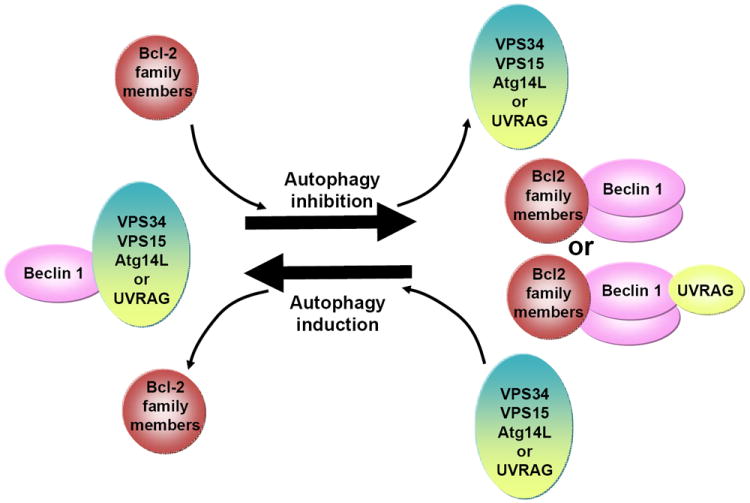
Beclin 1-VPS34-VPS15 forms pro-autophagic complexes with Atg14L or UVRAG. Binding of Bcl-2 and Beclin 1 is known to inhibit autophagy by possibly disrupting VPS34 binding; but Bcl-2 binding may also displace other stable interacting partners such as Atg14L (or UVRAG), resulting in autophagy inhibition. Additionally, the anti-autophagic binding of Bcl-2 promotes Beclin 1 homodimerization, which prevents heterodimerization with Atg14L or UVRAG.
