Abstract
Long-term culture of hepatocyte spheroids with high ammonia clearance is valuable for therapeutic applications, especially the bioartificial liver. However, the optimal conditions are not well studied. We hypothesized that liver urea cycle enzymes can be induced by high protein diet and maintain on a higher expression level in rat hepatocyte spheroids by serum-free medium (SFM) culture and coculture with mesenchymal stromal cells (MSCs). Rats were feed normal protein diet (NPD) or high protein diet (HPD) for 7 days before liver digestion and isolation of hepatocytes. Hepatocyte spheroids were formed and maintained in a rocked suspension culture with or without MSCs in SFM or 10% serum-containing medium (SCM). Spheroid viability, kinetics of spheroid formation, hepatic functions, gene expression, and biochemical activities of rat hepatocyte spheroids were tested over 14 days of culture. We observed that urea cycle enzymes of hepatocyte spheroids can be induced by high protein diet. SFM and MSCs enhanced ammonia clearance and ureagenesis and stabilized integrity of hepatocyte spheroids compared to control conditions over 14 days. Hepatocytes from high protein diet-fed rats formed spheroids and maintained a high level of ammonia detoxification for over 14 days in a novel SFM. Hepatic functionality and spheroid integrity were further stabilized by coculture of hepatocytes with MSCs in the spheroid microenvironment. These findings have direct application to development of the spheroid reservoir bioartificial liver.
Keywords: Bioartificial liver, Spheroid, Hepatocyte, Medium, Ureagenesis
INTRODUCTION
The bioartificial liver is a supportive system intended to provide hepatic detoxification to patients with acute liver failure during recovery of their native liver or until liver transplantation when recovery is unlikely (10). The bioartificial liver operates extracorporeally, like hemodialysis, but the bioartificial liver is unique in that it contains metabolically active liver cells capable of normal detoxification activity. Beneficial effects of bioartificial liver therapy have included improved survival and reduced neurological complications from subgroup analysis of two large randomized trials (6,16). These and other data suggest that further improvement in bioartificial liver therapy can be expected from increasing the dose of hepatocytes in the bioartificial liver. Therefore, we have developed a novel bioartificial liver composed of 40% of the hepatocyte mass of a normal human liver. The greater cell dose is accomplished with anchorage-independent aggregates of primary hepatocytes (“spheroids”) engineered by a novel rocked mixing technique. Once formed, spheroids are placed in a continuous perfusion bioreactor, the Spheroid Reservoir, which provides functionality to the device, the Spheroid Reservoir Bioartificial Liver (SRBAL) (14).
Our preliminary report of spheroid formation under rocked conditions was limited to a 5-day culture interval using porcine hepatocytes (20). More recently, spheroid formation kinetics of rat hepatocytes were compared in rocked versus rotational culture (2). These studies included characterization of gene expression and biochemical activity of rocker-formed rat hepatocyte spheroids over extended (14 days) duration. Our results indicate that the rocked technique is a simple yet rapid and highly efficient means of forming three-dimensional, suspension-stable, hepatic tissue constructs. Integrity and functionality of spheroids showed a gradual decline over 14 days under original conditions using a 10% serum-containing medium.
Recently, Nahmias et al. demonstrated enhanced functionality of monolayer-cultured hepatocytes when cultured in a serum-free medium and seeded under 95% oxygen conditions (11). More recently, our results indicate that normal atmospheric conditions provided the optimal oxygen tension for suspension culture of hepatocyte spheroids (12). It is now reasonable to ask whether serum-free medium will also enhance functionality and stabilize integrity of hepatocytes under spheroid conditions.
The morbidity of acute liver failure (ALF) is secondary to the tremendous decline in metabolic and synthetic functions inherent to the liver. With the decline in metabolic activity, accumulation of toxic substances occurs. The most notable of these toxins is ammonia. Cerebral edema (a life-ending complication of ALF) is strongly associated with elevated levels of ammonia (5). Thus, high urea cycle activity is essential for a liver support device such as the SRBAL in the treatment of ALF patients. High dietary protein intakes induce adaptive changes in levels of urea cycle enzyme activities (17). Recent reports demonstrate that coculture of mesenchymal stromal cells (MSCs) and hepatocytes significantly enhance hepatic functions compared to hepatocytes alone (24). Therefore, we hypothesize that coculture of MSCs and hepatocytes from animals fed a high protein diet would increase urea cycle activities and ammonia clearance, enhance other functionality, and stabilize spheroid integrity in long-term culture.
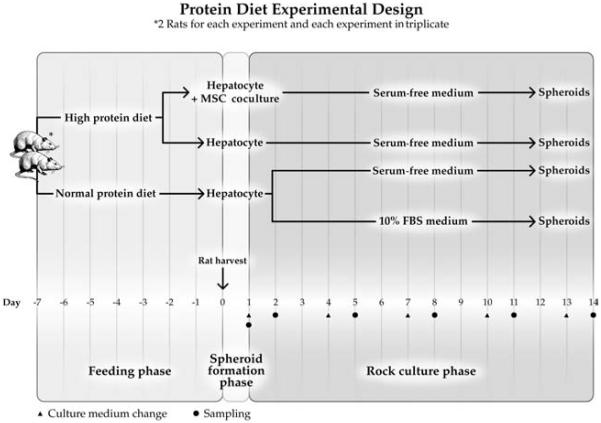
To test the hypothesis experimentally, we employed a xenogeneic model of rat hepatocytes in coculture with human MSCs. Before hepatocyte harvest, rats were feed standard or high protein diet (HPD) for 7 days. Spheroids were formed and maintained in rocked suspension culture. Spheroids were cultured in SFM or 10% serum medium to assess the influence of these variables on ammonia detoxification and other hepatic functions and integrity of hepatocytes as outlined in Figure 1.
Figure 1.
Experiment design. Rats were provided normal protein diet (NPD) or high protein diet (HPD) for 7 days before hepatocyte harvest. Freshly isolated hepatocytes were suspended in serum-free medium (SFM) or serum-containing medium (SCM) [Williams-E medium supplemented with 10% fetal bovine serum (FBS)] at a concentration 1 × 106 viable cells/ml. In heterotypic coculture system, hepatocytes (1 × 106/ml) were mixed with mesenchymal stromal cells (MSCs; 1 × 105/ml) and cultured in spheroid dishes. 20 ml suspended hepatocytes were inoculated into spheroid dishes (10 × 8 × 2 cm) and placed in the 37°C incubator with 5% CO2 and rocked continuously at 10 cycles per minute to induce spheroid formation and maintain suspension of spheroids. Culture medium was first changed at 24 h and subsequently at 3-day intervals. Dishes were sampled at 24 h after each medium change. Four culture groups were studied as follows: HPD + SFM + MSC group, hepatocytes harvested from high protein dietary rat and cultured in serum-free medium and cocultured with MSCs; HPD + SFM group, hepatocytes harvested from high protein dietary rat and cultured in serum-free medium; NPD + SFM group, hepatocytes harvested from normal protein dietary rat and cultured in serum-free medium; NPD + SCM group, hepatocytes harvested from normal protein dietary rat and cultured in serum-containing medium.
MATERIALS AND METHODS
Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Animals were housed in the Mayo Clinic vivarium and provided ad lib access to water and normal protein diet (NPD) (22% protein, Basal Diet 5755, TestDiet, Richmond, IN) or high protein diet (HPD) (60% Protein Purified Diet 5787, TestDiet, Richmond, IN) for 7 days before hepatocyte harvest. All animal procedures were performed under the guidelines set forth by the Mayo Foundation Animal Care and Use Committee in accordance with those set forth by the National Institutes of Health.
Rat Hepatocyte Isolation
Hepatocytes were isolated from 180 to 250 g male Sprague–Dawley rats (Harlan, Indianapolis, IN) by a two-step perfusion method as previously described (23). All harvests yielded hepatocytes with viability exceeding 95% by trypan-blue dye exclusion.
Mesenchymal Stromal Cells (MSCs)
Human MSCs were provided by the Mayo human cellular therapy laboratory. In brief, MSCs were isolated by collagenase enzyme digestion of human adipose tissue and cultured in 5% platelet lysate media [α-modified minimum essential medium (α-MEM) + GlutaMAX (200 mM/ml); 5% platelet lysate; 2 units/ml heparin]. MSCs were passaged at 60–80% confluence and seeded at 1 × 104/ml. All MSCs were studied before their fourth passage. All of the MSCs provided for this study came from the same donor. Adipose tissue used to produce human MSCs was collected as de-identified surgical waste material according to Mayo IRB procedures.
Spheroid Culture
Freshly isolated hepatocytes were suspended in serum-free medium (SFM) (Table 1) or serum-containing medium (SCM) [Williams-E medium supplemented with 10% fetal bovine serum (FBS; Mediatech, Inc., Herndon, VA), 10 U/ml penicillin G, 100 μg/ml streptomycin, 10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml sodium selenite] at a concentration of 1 × 106 viable cells/ml. Hepatocyte suspensions of 20 ml suspended hepatocytes were inoculated into spheroid dishes (10 × 8 × 2 cm) custom-made with glass and siliconized with Sigmacote. In coculture conditions, hepatocytes (1 × 106/ml) and MSCs (1 × 105/ml) were mixed immediately before inoculation into the spheroid dishes. Spheroid dishes were placed in the 37°C incubator with 5% CO2 and rocked continuously at 10 cycles per minute to induce spheroid formation and maintain suspension of spheroids.
Table 1.
Formula of Hepatocyte Spheroid Serum-Free Medium
| Component | Concentration |
|---|---|
| Williams' medium E | 10.8 g/L |
| Sodium bicarbonate | 2.2 g/L |
| Penicillin | 100 unit/ml |
| Streptomycin | 100 μg/ml |
| Insulin | 0.2 unit/ml |
| Porcine glucagon | 40 ng/ml (10 nM) |
| l-Glutamine | 2.0 mM |
| Human albumin | 3 g/L |
| Linoleic acid | 150 ng/ml |
| Mouse epidermal growth factor (EGF) | 5 ng/ml |
| Dexamethasone | 100 nM |
| l-Carnitine | 1 g/L |
| Glycine | 0.01 g/L |
| Human transferrin | 6 μg/ml |
| Gly-His-Lys | 20 ng/ml |
| CuSO4.5H2O | 0.1 μM |
| Na2SeO3 | 30 nM |
| ZnSO4.7H2O | 50 pM |
| Heparin | 1000 unit/L |
| Warfarin | 1 mg/L |
| l-Arginine | 0.2 g/L |
Culture Groups
Four culture groups were studied: HPD + SFM + MSC group, hepatocytes harvested from high protein dietary rat and cultured in serum-free medium and cocultured with MSCs; HPD + SFM group, hepatocytes harvested from high protein dietary rat and cultured in serum-free medium; NPD + SFM group, hepatocytes harvested from normal protein dietary rat and cultured in serum-free medium; NPD + SCM group, hepatocytes harvested from normal protein dietary rat and cultured in serum-containing medium.
Media Changes
Culture medium was first changed at 24 h and subsequently at 3-day intervals. One day prior to assays, culture medium was supplemented with 20 μM diazepam and 5% v/v heavy deuterium-enriched ammonia gas (2.23 mM; Cambridge Isotope Laboratories, Inc., Andover, MA) to assess the hepatic clearance of these compounds (20). Samples were also collected for measurement of albumin, diazepam, and urea.
Cell Counting
Representative 1-ml samples were removed from the spheroid dishes to determine spheroid diameter, spheroid number, and total hepatocyte volume (cell mass) using a Multisizer 3 (560 μm aperture; Beckman Coulter, Fullerton, CA).
Viability of Rat Hepatocytes
Spheroid viability were evaluated by inverted epi-fluorescent microscopy (Axioscope, Carl Zeiss, Inc., Thornwood, NY) using the Fluoroquench™ fluorescence viability stain (One Lambda, Canoga Park, CA). Fluorescence was quantified using Image J software from the NIH web site.
Real-Time RT-PCR
Total RNA was extracted from rat liver fed with NPD or HPD and from rat hepatocytes for each time point and group using an RNeasy Plus MiniKit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Use of this kit included the removal of residual genomic DNA. All PCR were carried out in 10-μl reaction volume using a QuantiFast SYBR Green RT-PCR kit (Qiagen) according to the manufacturer's instructions. All amplicons were intron spanning to rule out genomic DNA amplification (Table 2). Data were analyzed with the 7900HT Sequence Detection System (SDS) Version 2.3 software (Applied Biosystems, Foster City, CA) to estimate cycle threshold (Ct) values and enable observation of dissociation curves for characteristic melting curves and temperatures. Expression levels of each target gene were normalized to the expression levels of the endogenous reference gene hypoxanthine guanine phosphoribosyl transferase. The expression levels of each target gene in the day 0 NPD + SCM group of hepatocytes served as calibrators to determine the relative expression of each target gene at each time point and group.
Table 2.
RT-PCR Primer Sequences and Product Length
| Gene | Abbr. | Forward Primers | Reverse Primers | Size of Product |
|---|---|---|---|---|
| Albumin | Alb | GGCACCAAGTGTTGTACCCT | AGCACACACAGACGGTTCAG | 89 |
| Cytochrome P450c | Cyp1a1 | AGCTAATCAAAGAGCACTACAGG | CCTTATCATCTGAGAGCTGG | 128 |
| Cytochrome P450, family 1, subfamily A, polypeptide 2 | Cyp1a2 | GAGAAGGTGATGCTCTTCGG | ATGCAGGAGGATGGCTAAGA | 96 |
| Hepatocyte nuclear factor 4α | HNF4a | CCTTGGACCCAGCCTACA | GCTTGAGGCTCCGTAGTGT | 176 |
| Hepatocyte nuclear factor 6 | HNF6 | CCTGGAGCAAACTCAAGTCC | CCGTGTTCTTGCTCTTTCC | 127 |
| Ornithine transcarbamylase | Otc | TGAGGATCCTGCTCAACAAG | ACGGCCTTTCAGCTGTACTT | 107 |
| N-acetylglutamate synthase | NAGS | CCGTTCGGTGCTTCTAGACT | CAGGTTCACATTGCTCAGGA | 136 |
| Arginase 1 | Arg1 | CAACACTCCGCTGACAACC | CAGATATGCAGGGGGTCAC | 125 |
| Arginosuccinate lyase | Asl | TCAACAGTATGGATGCCACC | CAAAGTTGAATTCCTTGGTACC | 132 |
| Arginosuccinate synthetase | Ass1 | CCAGGAAGAAGGCACTGAAG | GCCTAGGAGATAGCGGTCCT | 131 |
| Carbamoyl-phosphate synthetase 1, mitochondrial | Cps1 | ACATTGGCTGCAGAATACCC | ACAGCCCAGCACCATTATTC | 108 |
| Hypoxanthine guanine phosphoribosyl transferase | Hprt | AGGACCTCTCGAAGTGTTGG | TCCACTTTCGCTGATGACAC | 138 |
Quantification of Ammonia and Isotopes of Urea
Production of urea through complete ureagenesis was determined by the conversion of heavy ammonia (15ND3) to native and deuterium-enriched urea. Isotopes of urea were quantified by capillary gas chromatography/mass spectrometry as previously reported (20,22).
Albumin and Urea Measurements
Measurements of urea were performed using a Quanti-Chrom™ DIUR-500 Urea Assay Kit (BioAssay System, Hayward, CA) according to the manufacturer's instructions. Albumin was measured with a rat albumin ELISA quantification kit from Bethyl Laboratories (Montgomery, TX) according to the manufacturer's instructions. All assays were done in triplicate.
Diazepam Metabolism
Concentrations of diazepam and its three major metabolites (nordiazepam, temazepam, oxazepam) were determined by high performance liquid chromatography (HPLC) with mass spectrometry detection as previously described (20). Duplicate samples were also analyzed after incubation in β-glucuronidase/arylsulfatase (600 Fishman units and 4800 Roy units/ml, respectively; Roche, Indianapolis, IN) and sodium acetate buffer (0.1 M) for 2 h. Reactions were quenched with ethanol. Data acquisition was completed by Chrom Perfect® software (Denville, NJ).
Cell Track
MSCs and hepatocytes were labeled using Cell-Tracker Kit (Molecular Probes, Eugene, OR) to identify their location in spheroid aggregates. MSCs were labeled green by CellTracker™ Green CMFDA (C2925), and hepatocytes were labeled red by CellTracker™ Red CMTPX (C34552).
Statistical Methods
Results were expressed as mean values ± SEM. Statistical analysis was done by one way ANOVA-Dunnett's test; a value of p = 0.05 or less was considered significant as compared to NPD + SCM group.
RESULTS
Develop a Specific SFM for Rat Hepatocyte Spheroids Culture
Our SFM was developed from a William's-E medium. Compared to previously reported SFM for rat hepatocyte culture (1,11,13), this SFM contained an antioxidant (1 g/L carnitine) and an increased concentration of human albumin (3 g/L). The formation of spheroids occurred in our SFM as fast as that in traditional 10% FBS William's-E media. Spheroids formed in our SFM with similar size and percentage of hepatocyte incorporation compared to traditional 10% FBS William's-E media. Higher albumin production and ureagenesis occurred using our SFM.
MSCs and SFM Stabilized the Hepatocyte Spheroids Integrity and Viability
We labeled MSCs green and hepatocytes red and observed the incorporation of MSCs into the hepatocyte aggregates (Fig. 2).
Figure 2.

MSCs and hepatocytes coculture. MSCs (green) and hepatocytes (red) were labeled using a CellTracker Kit. Left: Freshly isolated hepatocytes and MSCs (10:1) were suspended in spheroid dishes. Right: MSCs enrolled in the hepatocyte spheroids after 1-day of rocked culture. Scale bar: 50 μm.
In order to better understand the temporal decline in total biochemical activity of the spheroid cultures, we measured spheroid size, spheroid number, total cell volume, and percent incorporation of hepatocytes into spheroids (diameter > 60 μm) from the time of inoculation to day 14. These measurements were made in triplicate. All samples were obtained from the same glass plate conditions. We observed that about 75% of cells had incorporated into aggregates greater than 60-μm diameter by day 2 under both serum-free and 10% serum conditions. A noticeable decline in total cell number and spheroid number was observed in NPD + SCM group from day 2 to day 8. In contrast, these conditions remained relatively stable under HPD + SFM + MSC conditions till day 14 (Fig. 3A, B). Average spheroid size decreased from day 2 to day 14; spheroids in the HPD + SFM + MSC group decreased from 79.3 to 73.1 μm while spheroids in the NPD + SCM group decreased from 75.5 to 36.2 μm. Compared to average cell number on day 2 (100%), by day 14 the average cell number of the NPD + SCM group had declined to only 16.1% while average cell number of the HPD + SFM + MSC group remained relatively stable at 63.4%. Viability of hepatocytes in spheroids remained greater than 95% in all groups over the 14 days of culture. The HPD + SFM + MSC group maintained the highest viability, greater than 99%, at all time points (Fig. 3C).
Figure 3.
Hepatocyte count in suspension, incorporation into spheroids and viability. Cell counts were measured by Multisizer 3 Beckman Coulter counter. Cells were inoculated (day 0) at 1 × 106/ml. (A) Proportion of cells present in spheroids (>60 μm diameter). (B) Percent of hepatocyte mass left at each time point versus original cells inoculated of NPD + SCM. (C) Percent viability of cells in spheroids at each time point. Values represent the averages of three independent experiments. **p < 0.001; *p < 0.05 versus NPD + SCM at each time point.
Urea Cycle Enzymes Were Induced by High Protein Diet at the Pretranslational Level
We examined the expression of five liver-specific genes [hepatocyte nuclear factor 4a (HNF4a), HNF6, cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1a1), Cyp1a2, albumin] as well as the expression of six urea cycle genes [N-acetylglutamate synthase (Nags), carbamoyl-phosphate synthase 1 (Cps1), ornithine carbamoyltransferase (Otc), argininosuccinate synthase (Ass), argininosuccinate lyase (Asl), arginase (Arg1)] to evaluate hepatocyte functionality of the four different groups (Fig. 4). Expression levels were measured by qRT-PCR at five time points (days 0, 1, 2, 5, 14). The NPD + SCM group on day 0 served as the reference point. At harvest day 0, HPD groups had the highest expression levels of five of six urea cycle genes (Nags, Cps1, Otc, Ass, Asl) and two of five liver- specific genes (Cyp1a2, albumin). These values were 1.5- to 2.5-fold higher compared to NPD groups. By day 1, the expression levels of genes in HPD groups were similar to the level of the NPD groups. In contrast, the expressions of Cyp1a1 and HNF6 in the HPD and NPD groups were similar on day 0. However, their levels of expression were higher in the SFM groups compared to the SCM group at each time point. Gene expression was highest in the HPD + SFM + SCM group at all time points.
Figure 4.
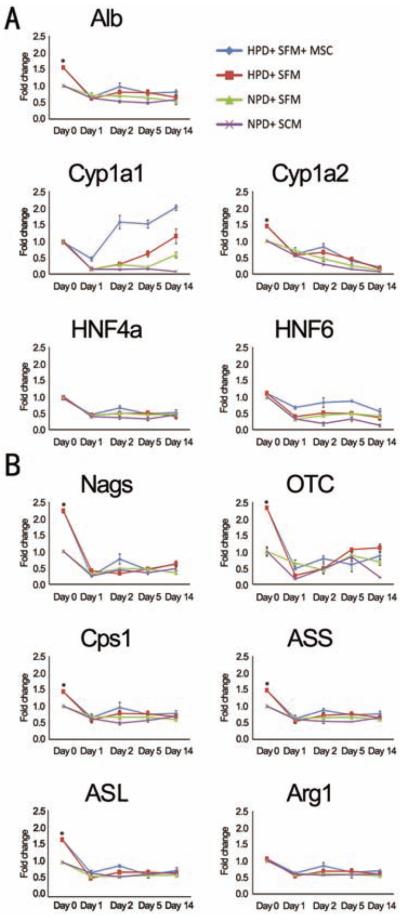
Expression of liver-specific genes. Gene expression of five liver-specific genes [albumin (Alb), Cyp1a1, Cyp1a2, HNF4a and HNF6] (A) and the six genes involved in the urea cycle (Nags, OTC, Cps1, ASS, ASL, and Arg1) (B) were examined by qRT-PCR during the 14-day time course for each of the four groups. NPD + SCM group day 0 values served as calibrators to determine the relative expression of each target gene at each time point and group. *p < 0.05 versus NPD groups at day 0. See Table 2 for gene definitions.
Metabolic Functions Improved by High Protein Diet and Serum-Free Medium
Functionality of hepatocyte spheroids was determined in each of the four groups by the production of albumin and urea (Fig. 5) and diazepam metabolism (Fig. 6).
Figure 5.
Albumin and urea production. (A) Measurement of rat albumin by ELISA. (B) Average urea production for each group was determined by a commercially available QuantiChrom™ DIUR-500 urea assay kit. (C) Heavy ammonia (0.5% vol/vol) was added to culture media and levels of deuterium-enriched heavy urea were determined by gas chromatography/mass spectrometry on samples collected at 24 h. (A–C) Left: Results are normalized to volumes of media. Right: Results are normalized to volumes of media and cell number, and p values between each group at each time point. (D) Ammonia concentration in the samples was determined after 24 h and reported as the percent of ammonia clearance. **p < 0.001; *p < 0.05 versus NPD + SCM at each time point.
Figure 6.
Diazepam metabolism. (A) 20 μM diazepam was added to culture media, and diazepam elimination was calculated on samples collected at 24 h for each group at each time point. **p < 0.001; *p < 0.05 versus NPD + SCM at each time point. (B) Samples were incubated, or not, in β-glucuronidase/arylsulfatase in sodium acetate buffer for 2 h. Reactions were quenched with ethanol. Concentrations of diazepam metabolite formation were determined by high-performance liquid chromatography (HPLC) with mass spectrometry detection. The concentration of conjugated temazepam and conjugated oxazepam, markers of phase 2 metabolism, were determined for each group at each time point.
Albumin Production
By ELISA, we observed that production of albumin protein was significantly higher in both HPD groups compared to the NPD groups (p < 0.001) at day 1. Albumin production was significantly higher in SFM groups compared to the SCM group from day 2 to day 14 (p < 0.001) (Fig. 5A). The MSCs provided a relative stability on the production of albumin.
Ammonia Clearance
The ammonia clearance of NPD + SCM group declined rapidly during the second week in culture (from 99.5% to 55.6%), while ammonia clearance of HPD + SFM + MSC was stable (from 98.9% to 85.7%) during the second week of culture (Fig. 5B).
Urea Cycle
The trend in urea production was similar to the trend in albumin production for each of the four groups (Fig. 5C). All groups demonstrated significant levels of ureagenesis by the conversion of deuterium-enriched (heavy) ammonia to isotopes of heavy urea at all time points. The proportion of heavy urea to total urea produced by spheroid hepatocytes in all groups fluctuated from 39.8% to 42.1% (Fig. 5D).
Diazepam Metabolism
Cyp1a1 gene expression increased significantly at all time points in SFM groups. Notably, Cyp1a1 gene expression was highest in HPD + SFM + MSC group on day 14. The elimination of diazepam and the ability of cultured rat hepatocytes to convert diazepam to its major metabolites (nordiazepam, temazepam, and oxazepam) were used to assess cytochrome p450 activity. Diazepam elimination was approximately 100% of all groups at day 1 and day 2, but decreased persistently from day 5 to day 14 (Fig. 6A). NPD + SCM group declined fastest from 44.7% at day 5 to 13.9% at day 14. However, HPD groups kept relatively stable from 86.4% at day 5 to 53.7% at day 14. The phase II metabolism activities were assessed by the levels of conjugated temazepam and conjugated oxazepam. In the first 2 days, all groups produced very little conjugated temazepam and conjugated oxazepam. However, from day 5 to day 14, SFM groups had significantly higher levels of conjugated temazepam and conjugated oxazepam compared to the SCM group (Fig. 6B) indicating better maintenance of phase 2 metabolism by hepatocyte spheroids under serum-free conditions.
DISCUSSION
The liver is one of first lines of defense and the main detoxifying organ of the body. Ammonia is arguably the most important toxin which requires clearance by the liver (7). Ammonia accumulation correlates with the most feared complication of the acutely failing liver, cerebral edema (9). For ammonia to be effectively detoxified and eliminated by the body, a functioning urea cycle must be present (27). Similarly, urea production and ammonia removal are important activities of a life-saving bioartificial liver device (15). Primary hepatocytes have demonstrated therapeutic importance in extracorporeal bioartificial liver devices and in cell transplant treatments (26). A massive demand exists for hepatocytes with regards to quantity and quality. These demands have not been sufficiently met with current culture techniques despite much effort and some progress towards this end. Efforts to improve bioartificial liver therapy continue to address the microenvironment and cellular architecture within the device to optimize long-term functionality.
One such system, suspension culture of spheroid hepatocytes, is described in this work. In preliminary experiments we tested “serum-free medium (SFM) versus serum-contained medium (SCM),” “normal protein (20%) diet (NPD) versus medium protein (40%) diet (MPD) versus high protein (60%) diet (HPD),” and “coculture with a series of ratios of MSCs/hepatocytes (1:3, 1:5, 1:10, 1:20 and 0:20)”. Our preliminary studies demonstrated that serum-free medium was superior to medium containing serum for culture of rat hepatocytes; high protein diet induced the highest urea cycles enzymes activities in rat hepatocytes; stability, integrity, and viability of hepatocyte spheroids were best when MSC/hepatocyte mixtures were cocultured at the ratio of 1:10 for 14 days. From these preliminary univariate studies, we concluded that “SFM,” “HPD,” and “MSC (1:10)” showed greatest benefit and should be studied in combination. In this study, we investigated the synergism of SFM, MSC, and HPD in a hepatocyte spheroid culture system. We demonstrate that the combination of a high protein diet, a serum-free culture medium, and MSC coculture supports a remarkable level of liver specific functionality, including ammonia detoxification, gene expression, cell viability, and integrity of rat hepatocyte spheroids.
A significant element of our system is the SFM formulation. The use of SFM represents an important tool in cell culturing. SFM allows for a more tightly controlled culturing environment with less confounding variables. Hormonally defined medium has been reported to support gene transcription and gap junction communication in primary rat hepatocytes (4,8). However, past SFM were not suitable for efficient formation of spheroids (3,18,21). Our SFM was developed based on William's-E media using our novel rocked culture system. Rocking maximizes the frequency of cell-to-cell contact and rapidly enhances the rate and efficiency of spheroid formation from isolated hepatocytes. Our SFM contained 1g/L carnitine and an increased concentration of human albumin (3 g/L), which was similar to the normal physiological concentration of albumin in blood. The formation of spheroids in our SFM was as fast as in traditional SCM. Spheroids formed in our SFM were similar in size and percentage incorporation of hepatocytes to SCM. Higher rates of albumin production and ureagenesis were also observed using our SFM. Our SFM continuously enhanced higher functionality and stabilized integrity of spheroids with better viability than SCM for 14 days. In addition, our SFM also supported high cell density culture of primary rat hepatocytes (5 × 106 cell/ml) similar to traditional SCM. Our data suggests that cellular attachment was enhanced by higher albumin concentration in our SFM. Furthermore, the absence of serum resulted in improved functionality suggesting the presence of inhibitory substances in serum.
The mRNA levels of five of six urea cycle enzymes increased in rats during high dietary protein intake before hepatocyte isolation. The mRNA levels of these five urea cycle genes declined in culture similar to the levels of NPD groups, the higher rate of urea production remained relatively stable despite a late fall-off in urea cycle genes expression. We hypothesized that a high protein diet to donor rats would lead to increased levels of urea cycle enzymes at the time of hepatocyte isolation. Since the half-life of urea cycle enzymes was longer than the corresponding mRNA levels, the urea cycle activity of hepatocytes from rats fed a high protein diet was significantly higher for an extended duration than hepatocytes from rats fed a normal protein diet. This pattern represents a lifespan of continued enzyme activity even after loss of mRNAs (17,25).
Ammonia is arguably the most important toxin requiring clearance by the liver in acute liver failure. Accumulation of ammonia correlates with formation of cerebral edema, the most feared complication of acute liver failure. A functional urea cycle is crucial to detoxification of ammonia. In our studies, a high protein diet before hepatocyte isolation induced the expression of urea cycle genes, raised urea production, and decreased ammonia levels. Though as yet unproven, we hypothesize that a high level of ammonia clearance is vital to successful application of an extracorporeal liver assist device. Lowering blood ammonia is vital to halting the progression of cerebral edema and allowing time for recovery or in bridging patients with acute liver failure to liver transplantation. A rapid decline in blood ammonia is associated with successful liver transplantation, including patients with acute liver failure.
Cocultured of hepatocytes with other cell types has been shown to mimic the liver-like microenvironment (13,19). In our study, MSCs participated in spheroid formation and stabilized the integrity of spheroids. Coculture also enhanced the hepatic functions of hepatocytes for 14 days. These benefits may be the result of high levels of extracellular matrix and the role of soluble factors derived from the supporting cells (24).
In conclusion, these results demonstrated that our newly developed SFM appears suitable for application in cell culturing models utilizing spheroids formed from hepatocytes. The hepatocytes from rats after high protein intake had higher urea cycle activities. The coculture system displayed functional advantages over a pure hepatocyte-based system. The combination of these three factors was associated with improved health and vital function of hepatocyte spheroids under rocked suspension culture conditions. These results indicate that a serum-free medium should be used preferentially in a continuous perfusion bioreactor such as the Spheroid Reservoir, an apparatus which provides functionality to the Spheroid Reservoir Bioartificial Liver (SRBAL) for treatment of liver failure.
ACKNOWLEDGMENTS
This work was funded by the Marriott Foundation, NIH, and China Scholarship Council.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Abu-Absi S, Friend J, Hansen L, Hu W-S. Structural polarity and functional bile canaliculi in rat hepatocytes spheroids. Exp. Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- 2.Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, Nyberg SL. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49:578–586. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong TW, Smith RL, Hughes MG, Camden J, Rudy CK, Evans HL, Sawyer RG, Pruett TL. Primary human hepatocytes in spheroid formation to study hepatitis C infection. J. Surg. Res. 2006;130:52–57. doi: 10.1016/j.jss.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Chua K, Lim W, Zhang P. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–2547. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Clemmesen J, Larsen F, Kondrug J, Hansen B, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- 6.Demetriou AA, Brown Jr., R. S., Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS, 2nd., Lerut J, Nyberg SL, Salizzoni M, Fagan EA, de Hemptinne B, Broelsch CE, Muraca M, Salmeron JM, Rabkin JM, Metselaar HJ, Pratt D, De La Mata M, McChesney LP, Everson GT, Lavin PT, Stevens AC, Pitkin Z, Solomon BA. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann. Surg. 2004;239:660–667. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis J, Delorme ML, Boschat M, Nordlinger B, Opolon P. Respective roles of ammonia, amino acids, and medium-sized molecules in the pathogenesis of experimentally induced acute hepatic encephalopathy. J. Neurochem. 1983;40:10–19. doi: 10.1111/j.1471-4159.1983.tb12646.x. [DOI] [PubMed] [Google Scholar]
- 8.Glicklis R, Merchuk JC, Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol. Bioeng. 2004;86:672–680. doi: 10.1002/bit.20086. [DOI] [PubMed] [Google Scholar]
- 9.Haussinger D, Lamers W, Moorman A. Hepatocyte heterogeneity in the metabolism of amino acids and ammonia. Enzyme. 1992;46:72–93. doi: 10.1159/000468779. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt W, Chen S, Watanabe W, Arnaout W, Shackleton C, Rozga J, Demetriou A. Treatment of acute hepatic failure with porcine hepatocyte-based bioartificial liver (BAL) Hepatology. 1996;24:436A. [Google Scholar]
- 11.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc. Natl. Acad. Sci. USA. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lillegard JB, Fisher JE, Nedredal G, Luebke-Wheeler J, Bao J, Wang W, Amoit B, Nyberg SL. Normal atmospheric oxygen tension and the use of antioxidants improve hepatocyte spheroid viability and function. J. Cell. Physiol. 2011;226:2987–2996. doi: 10.1002/jcp.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu HF, Chua KN, Zhang PC, Lim WS, Ramakrishna S, Leong KW, Mao HQ. Three-dimensional coculture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta Biomater. 2005;1:399–410. doi: 10.1016/j.actbio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh M, Corner S, Amiot B, Nyberg S. Engineering analysis and development of the spheroid reservoir bioartificial liver. IEEE Eng. Med. Biol. Soc. 2009;2009:5985–5988. doi: 10.1109/IEMBS.2009.5334687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millis J, Losanoff J. Technology insight: Liver support systems. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:398–405. doi: 10.1038/ncpgasthep0254. [DOI] [PubMed] [Google Scholar]
- 16.Millis JM, Cronin DC, Johnson R, Conjeevaram H, Conlin C, Trevino S, Maguire P. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: System modifications and clinical impact. Transplantation. 2002;74:1735–1746. doi: 10.1097/00007890-200212270-00016. [DOI] [PubMed] [Google Scholar]
- 17.Morris SM, Jr., Moncman CL, Rand KD, Dizikes GJ, Cederbaum SD, O'Brien WE. Regulation of mRNA levels for five urea cycle enzymes in rat liver by diet, cyclic AMP, and glucocorticoids. Arch. Biochem. Biophys. 1987;256:343–353. doi: 10.1016/0003-9861(87)90455-3. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa K, Lee SW, Fukuda J, Yang DH, Kunitake T. Hepatocyte spheroid formation on a titanium dioxide gel surface and hepatocyte long-term culture. J. Mater. Sci. Mater. Med. 2006;17:359–364. doi: 10.1007/s10856-006-8237-7. [DOI] [PubMed] [Google Scholar]
- 19.Nedredal GI, Elvevold K, Ytrebo LM, Fuskevag OM, Pettersen I, Bertheussen K, Langbakk B, Smedsrod B, Revhaug A. Significant contribution of liver nonparenchymal cells to metabolism of ammonia and lactate and cocultivation augments the functions of a bioartificial liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G75–G83. doi: 10.1152/ajpgi.00245.2006. [DOI] [PubMed] [Google Scholar]
- 20.Nyberg SL, Hardin J, Amiot B, Argikar UA, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transplant. 2005;11:901–910. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 21.Peshwa M, Wu F, Follstad B, Cerra F, Hu W-S. Kinetics of hepatocyte spheroid formation. Biotechnol. Prog. 1994;10:460–466. [Google Scholar]
- 22.Rinaldo P, Hahn S, Matern D. Inborn errors of amino acid, organic acid, and fatty acid metabolism. In: Burtis C, Ashwood E, Bruns D, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. Elsevier Saunders; St. Louis, MO: 2005. pp. 2207–2247. [Google Scholar]
- 23.Seglen P. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 24.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin. Exp. Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snodgrass P, Lin R, Muller W, Aoki T. Induction of urea cycle enzymes of rat liver by glucagon. J. Biol. Chem. 1978;253:2748–2753. [PubMed] [Google Scholar]
- 26.Tsiaoussis J, Newsome P, Nelson L, Hayes P, Plevris J. Which hepatocyte will it be? Hepatocyte choice for bioartificial liver support systems. Liver Transplant. 2001;7:2–10. doi: 10.1053/jlts.2001.20845. [DOI] [PubMed] [Google Scholar]
- 27.Ytrebo LM, Kristiansen RG, Maehre H, Fuskevag OM, Kalstad T, Revhaug A, Cobos MJ, Jalan R, Rose CF. L-ornithine phenylacetate attenuates increased arterial and extracellular brain ammonia and prevents intracranial hypertension in pigs with acute liver failure. Hepatology. 2009;50:165–174. doi: 10.1002/hep.22917. [DOI] [PubMed] [Google Scholar]