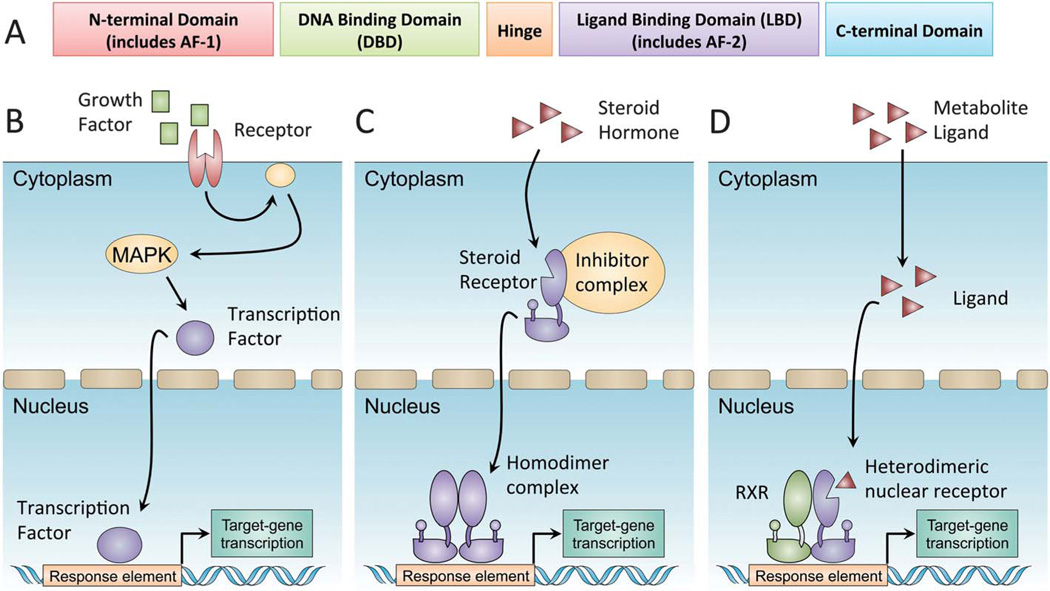
Fig. 1.
Nuclear receptor transcriptional activation. Nuclear receptors are a family of ligand-activated transcription factors. (A) Members of the nuclear receptor superfamily have a common domain structure consisting of an amino-terminal activation domain (AF-1), a DNA-binding domain, and a carboxy-terminal ligand-binding domain (LBD). The LBD determines ligand-regulated interactions with co-activators and co-repressors through allosteric changes in a short helical region known as AF-2. (B) In a canonical signal-transduction cascade, receptor binding at the plasma membrane initiates enzymatic phosphorylation cascades culminating with transcription factor translocation into the nucleus. (C) Type I steroid nuclear receptors are synthesized in inactive forms associated with heat-shock protein (HSP) complexes in the cytoplasm. Direct hormone binding causes a conformational change, dissociation from HSP complexes and translocation into the nucleus. (D) Type II heterodimeric nuclear receptors bind constitutively to DNA with RXRs as obligate partners. Ligand binding causes a conformational change, dissociation of co-repressor complexes and recruitment of co-activators, such as PGC1α.