Abstract
The aryl hydrocarbon receptor (AHR)–dependent induction of cytochromes P450 (P450) such as CYP1A1 by 3-methylcholanthrene (MC) and related polycyclic aromatic hydrocarbons is well characterized. We reported previously that MC treatment triggers a pronounced downregulation, particularly at the protein level, of mouse hepatic Cyp3a11, a counterpart of the key human drug-metabolizing enzyme CYP3A4. To determine whether this effect of MC requires hepatic microsomal P450 activity, we studied liver Cpr-null (LCN) mice with hepatocyte-specific conditional deletion of the NADPH-cytochrome P450 oxidoreductase gene. In vehicle-treated animals, basal levels of CYP3A11 mRNA and CYP3A protein immunoreactivity were elevated by approximately 9-fold in LCN mice compared with wild-type (WT) mice, whereas CYP3A catalytic activity was profoundly compromised in LCN mice. MC treatment caused suppression of CYP3A11 mRNA, CYP3A protein immunoreactivity, and CYP3A catalytic activity in WT mice, and the MC effects at the mRNA and protein levels were maintained in LCN mice. Flavin-containing monooxygenase-3 (Fmo3) induction by MC was suggested previously to occur via an AHR-dependent mechanism requiring conversion of the parent compound to DNA-damaging reactive metabolites; however, hepatic FMO3 mRNA levels were dramatically increased by MC in both WT and LCN mice. MC did not function as a mechanism-based inactivator of CYP3A enzymes in hepatic microsomes prepared from untreated WT mice, under conditions in which 1-aminobenzotriazole caused marked NADPH-dependent loss of total P450 content and CYP3A catalytic activity. These results indicate that MC downregulates mouse hepatic CYP3A protein via a pretranslational mechanism that does not require hepatic microsomal P450-dependent activity.
Introduction
Human CYP3A4, an abundant cytochrome P450 (P450) enzyme in liver and intestine, metabolizes numerous drugs and endogenous chemicals. Little is known about the regulation of CYP3A4 and its murine counterparts by the aryl hydrocarbon receptor (AHR), the key mediator of adaptive and toxic responses to halogenated aromatic hydrocarbons (HAHs) such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons (PAHs) such as 3-methylcholanthrene (MC). Although MC treatment decreases CYP3A4 expression and activity in primary human hepatocytes (Richert et al., 2009) and smokers display decreased CYP3A4-mediated conversion of ropivacaine to (S)-2′,6′-pipecoloxylidide (Jokinen et al., 2001), both in vitro (He et al., 2006) and in vivo (Kassai et al., 1988) measures of midazolam clearance reveal no differences between smokers and nonsmokers.
The mouse Cyp3a subfamily includes eight members, with Cyp3a11 encoding the most abundant hepatic CYP3A protein in male mice (Yanagimoto et al., 1997). TCDD, an essentially nonmetabolized AHR agonist, causes AHR-dependent suppression of hepatic CYP3A11 (Lee and Riddick, 2012) and CYP3A13 (Ovando et al., 2006) mRNA levels. Proteomic analysis showed that MC, a readily metabolized AHR agonist, decreases mouse hepatic CYP3A11/16/41 protein levels (Jenkins et al., 2006) and we found that the decrease in mouse hepatic Cyp3a11 expression caused by MC was particularly pronounced at the protein level (Lee et al., 2006).
MC is biotransformed by constitutive and inducible P450s into several oxidative metabolites, some of which are reactive and toxic (Wood et al., 1978). Parent PAHs strongly inhibit the P450s involved in PAH bioactivation (Shimada and Guengerich, 2006) and MC can be converted to reactive metabolites that bind covalently to microsomal proteins including P450s (Tunek et al., 1979). The rapid and pronounced loss of mouse hepatic CYP3A protein seen in response to MC treatment (Lee et al., 2006) suggested that MC may be metabolically activated to reactive products triggering CYP3A protein destruction. However, it was important to use a metabolism-deficient genetically modified mouse model to determine whether this MC response is due to the parent compound or the generation of metabolites.
The goals of this study were to determine whether suppression of Cyp3a11 by MC requires metabolism by hepatic microsomal P450s, and whether MC causes mechanism-based inactivation of mouse hepatic microsomal CYP3A enzymes. We studied mice that are essentially devoid of hepatic microsomal P450 activity due to hepatocyte-specific conditional deletion of the NADPH–cytochrome P450 oxidoreductase (Cpr or Por) gene, which encodes the key electron transfer partner for all microsomal P450s. These liver Cpr-null (LCN) mice also show compensatory increased expression of several P450 mRNAs/proteins and hepatic lipidosis (Gu et al., 2003).
Materials and Methods
Treatment of LCN Mice.
Details of the study on LCN mice were described previously (Lee et al., 2013) and a brief summary of the animal work is given here for context. This study used adult male (aged 8–9 weeks) LCN mice (Alb-Cre +/−/Por lox/lox) (Gu et al., 2003) and wild-type (WT) littermates (Alb-Cre −/−/Por lox/lox), both on a C57BL/6 background, with approval from the Institutional Animal Care and Use Committee of the Wadsworth Center. Groups of four LCN and WT mice received a single intraperitoneal injection of MC (80 mg/kg) or an equivalent volume of corn oil vehicle. At 72 hours after injection, mice were euthanized and harvested liver tissue was frozen and stored for subsequent isolation of total RNA and microsomes.
Analysis of mRNA Levels by Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction.
Hepatic levels of mRNAs encoding CYP3A11 and flavin-containing monooxygenase-3 (FMO3), normalized to β-actin as the internal reference standard, were determined by the comparative threshold cycle relative quantitation method on the ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). The CYP3A11 primers and optimized assay conditions were validated in our previous study (Lee and Riddick, 2012), whereas the FMO3 primer sequences were derived from published work (Zhang et al., 2009).
Immunoblot Analysis.
General procedures were described previously (Lee et al., 2006). Blots derived from gels originally loaded with 5 µg hepatic microsomal protein were probed with a rabbit polyclonal antibody against rat CYP3A2, dilution 1:50,000 (BD Gentest, Bedford, MA).
Luciferin Isopropyl Acetal Activity.
Luciferin isopropyl acetal (Luc-IPA) is a highly specific probe substrate for human CYP3A4 (Cali et al., 2012), and we have used it as an index of mouse CYP3A catalytic activity since in vivo bioluminescence imaging and in vitro assays with mouse liver homogenate show strong induction of this activity by dexamethasone (Roncoroni et al., 2012). Using a P450-Glo Assay (Promega, Madison, WI), liver microsomes (0.8 µg protein) were incubated with Luc-IPA for 30 minutes followed by incubation with the NADPH regeneration system for 30 minutes. Luciferin detection reagent (25 µl) was added to each 25-µl reaction mixture, and luminescence was read 30 minutes later using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Luminescence of a control reaction lacking microsomes was subtracted from each sample, and activities were normalized for microsomal protein content. All steps took place at room temperature, and the final concentration of Luc-IPA in the enzyme reactions was 3 µM.
MC as a Potential Mechanism-Based Inactivator of Mouse CYP3A.
Adult male (aged 8–9 weeks) C57BL/6 mice from Charles River Canada (St. Constant, QC, Canada) were used in a study approved by the University of Toronto Animal Care Committee. After cervical dislocation, four independent pools of hepatic microsomes (each pool derived from 18 individual mice) were prepared by standard differential centrifugation techniques.
Hepatic microsomes (9–12 mg protein) from untreated mice were incubated in 6-ml reactions in 0.1 M potassium phosphate buffer, pH 7.4, containing 1.5 mM EDTA in the presence of MC (0.1, 1, 5, or 10 µM) and NADPH (1 mM) for 30 minutes at 37°C in a shaking water bath. The positive control 1-aminobenzotriazole (ABT) (1 mM) replaced MC in some reactions. The following controls were run: omission of NADPH and inactivator, omission of inactivator, and omission of NADPH. MC and ABT were dissolved in dimethylsulfoxide, with the final solvent concentration being 0.5%. Reactions were terminated by cooling samples on ice.
After this initial incubation phase, microsomes were reharvested by centrifugation to remove residual inactivator and thereby minimize competitive enzyme inhibition. Total P450 content was determined by the reduced-carbon monoxide difference spectrum method as described (Lee et al., 2006). CYP3A immunoreactivity and Luc-IPA activity were assessed as above. For POR immunoreactivity, blots derived from gels originally loaded with 4 µg hepatic microsomal protein were probed with a rabbit polyclonal antibody against human POR, dilution 1:2000 (Santa Cruz Biotechnology Inc., Santa Cruz, CA). POR activity was assessed as the rate of cytochrome c reduction as described (Lee et al., 2013).
Statistical Analysis.
Data are expressed as the mean ± S.D. of determinations from four mice or four microsomal pools. Statistical analyses were performed on the original raw data and not on the percentage of control data presented in the figures and some table columns. A result was considered statistically significant at P < 0.05.
For the in vivo MC treatment study, data were analyzed initially using a randomized-design two-way analysis of variance (ANOVA) to identify significant influences of the two independent variables and their interaction (treatment, genotype, treatment × genotype interaction). Post-test analyses for the planned comparisons (treatment effect, genotype effect) were performed to assess whether there were significant differences between particular groups. Post tests were Bonferroni corrected for multiple comparisons and used the mean square residual (pooled variance) and corresponding degrees of freedom from the two-way ANOVA. In cases in which Bartlett’s test revealed significant heterogeneity of variance (CYP3A11 mRNA, FMO3 mRNA, CYP3A protein), specific comparisons of interest were made using the nonparametric Welch-corrected unpaired t test. The magnitude of the MC effect in WT versus LCN mice was compared using the t test.
For the in vitro investigation of MC as a potential mechanism-based inactivator, control and treatment measurements from matched microsomal pools were compared using a repeated-measures design one-way ANOVA followed by a post hoc Newman–Keuls test.
Results and Discussion
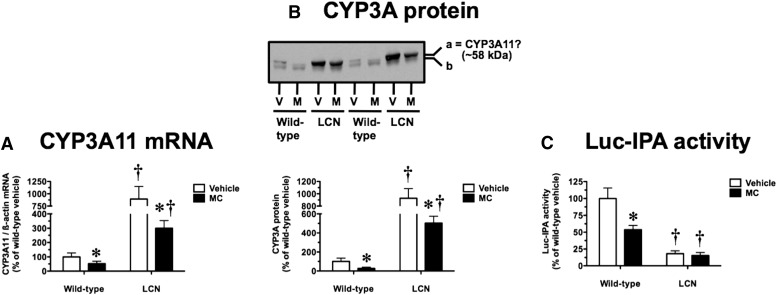
An MC dosing regimen shown previously to cause robust induction of AHR target genes in WT and LCN mice (Lee et al., 2006, 2013) decreased hepatic CYP3A11 mRNA levels by 48 and 66% in WT and LCN mice, respectively (Fig. 1A); the magnitude of this response did not differ between mouse genotypes (P = 0.4327). In vehicle-treated mice, the basal CYP3A11 mRNA levels were 8.9-fold higher in LCN mice compared with WT mice (Fig. 1A). As in our earlier work (Lee et al., 2006), we assessed CYP3A protein levels using a rabbit polyclonal antibody raised against rat CYP3A2, which recognizes two protein bands (labeled “a” and “b” in Fig. 1B) in mouse liver microsomes. Focusing on the more prominent upper band “a”, thought to represent CYP3A11 (Katoh et al., 2005), we found that MC decreased CYP3A immunoreactivity by 73 and 46% in WT and LCN mice, respectively (Fig. 1B); the magnitude of this response did not differ between mouse genotypes (P = 0.2400). The basal CYP3A protein signal intensity was 9.3-fold higher in LCN mice compared with WT mice (Fig. 1B). At the functional level, we used Luc-IPA as a substrate to assess the activity of CYP3A enzymes, microsomal P450s that depend on POR for electron transfer. In vehicle-treated mice, the basal Luc-IPA activity of LCN mice was 18% of that seen in WT mice (Fig. 1C). This activity was decreased by 46% in WT mice after MC treatment, but MC had no effect on the already low Luc-IPA activity in LCN mice (Fig. 1C).
Fig. 1.
Effect of MC treatment on hepatic CYP3A11 mRNA (A), CYP3A protein immunoreactivity (B), and Luc-IPA catalytic activity (C) in WT and LCN mice. (B) Immunoblot of microsomal protein (5 µg) using polyclonal antibody against rat CYP3A2, showing results for two vehicle (V)–treated and MC (M)–treated mice per genotype. This antibody recognizes two mouse protein bands, as shown by the “a” and “b” labels on the right side, with the more prominent upper band “a” thought to represent CYP3A11. Quantitative analysis of CYP3A11 mRNA levels, relative to β-actin (A), CYP3A protein immunoreactivity levels as reflected by band “a” intensity (B), and Luc-IPA activity (C). Data represent the mean ± S.D. of determinations from four mice per group, expressed as a percentage of the mean for the vehicle-treated WT mice. The P values for the two-way ANOVA main effects for the Luc-IPA data were P = 0.0001 (treatment), P < 0.0001 (genotype), and P = 0.0004 (interaction). Outcomes from nonparametric Welch-corrected unpaired t tests (CYP3A11 mRNA and CYP3A protein immunoreactivity, where heterogeneity of variance was detected) and Bonferroni-corrected post tests (Luc-IPA activity) were as follows: *significantly different (P < 0.05) from genotype-matched vehicle-control mice; †significantly different (P < 0.05) from treatment-matched WT mice.
Consistent with these findings, LCN mice were previously shown to have a 11-fold elevation of CYP3A11 mRNA levels (Gu et al., 2003) and a marked increase in CYP3A protein (Gu et al., 2003; Weng et al., 2005). The similar hepatic P450 reductase-null (HRN) mouse strain has a 7.7-fold elevation of CYP3A11 mRNA levels (Wang et al., 2005) and a marked increase in CYP3A protein (Henderson et al., 2003). Increased basal expression of CYP3A11 mRNA and CYP3A protein in the LCN and HRN mice is thought to be due primarily to activation of the constitutive androstane receptor (Weng et al., 2005) by accumulated unsaturated fatty acids (Finn et al., 2009). Despite the pronounced elevation of CYP3A11 mRNA and CYP3A protein, we confirmed that CYP3A catalytic activity is profoundly compromised in LCN mice due to loss of POR-dependent electron transfer to microsomal P450s, as reported previously for both LCN (Gu et al., 2003) and HRN (Henderson et al., 2003) mice.
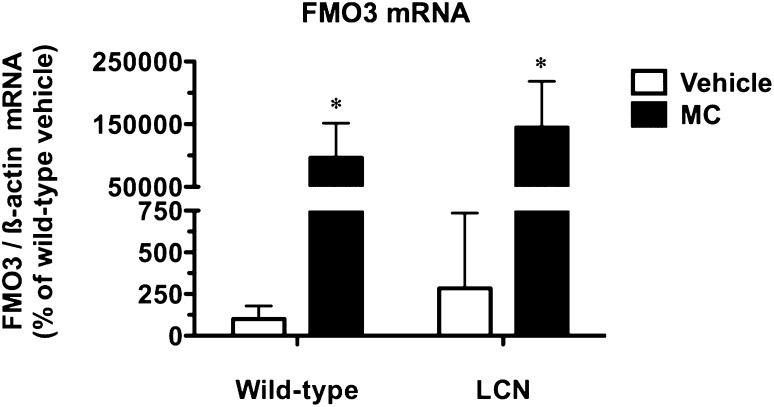
We also studied the regulation of a hepatic gene postulated to show preferential induction after biotransformation of PAH-type inducers. Readily metabolized PAHs such as MC induce FMO3 mRNA levels in mouse liver and Hepa-1 mouse hepatoma cells and the following mechanism was proposed: AHR activation by PAHs causes induction of CYP1 enzymes that convert the parent compounds into DNA-damaging reactive metabolites, thereby leading to Fmo3 induction via activation of the p53 response pathway (Celius et al., 2010). However, MC dramatically increased hepatic FMO3 mRNA levels by 964- and 510-fold in WT and LCN mice, respectively (Fig. 2); the magnitude of this response did not differ between mouse genotypes (P = 0.5010). The basal FMO3 mRNA levels did not differ between WT and LCN mice (Fig. 2). The maintenance of the Fmo3 induction response in LCN mice versus WT mice suggests that the production of DNA-damaging reactive metabolites, if required, may occur through the actions of non-P450 enzymes and/or in cell types other than hepatocytes.
Fig. 2.
Effect of MC treatment on hepatic FMO3 mRNA in WT and LCN mice. Quantitative analysis of FMO3 mRNA levels, relative to β-actin. Data represent the mean ± S.D. of determinations from four mice per group, expressed as a percentage of the mean for the vehicle-treated WT mice. Outcomes from nonparametric Welch-corrected unpaired t tests (since heterogeneity of variance was detected for FMO3 mRNA) were as follows: *significantly different (P < 0.05) from genotype-matched vehicle-control mice.
Using hepatic microsomes from untreated WT mice, we tested the hypothesis that MC is a mechanism-based inactivator of mouse CYP3As resulting in irreversible loss of catalytic activity and accelerated proteolytic degradation. The positive control was ABT, which causes well characterized mechanism-based inactivation of multiple P450s via formation of an NN-bridged benzyne-protoporphrin IX adduct (Ortiz de Montellano and Mathews, 1981). Incubation of mouse liver microsomes with ABT for 30 minutes demonstrated marked NADPH-dependent P450 inactivation, with ABT lowering total P450 content to 36% and Luc-IPA activity to 3% of the levels observed after incubation with NADPH alone (Table 1). As expected, incubation with ABT did not alter microsomal CYP3A protein levels, POR protein levels, or POR-mediated cytochrome c reduction activity (Table 1). Concentrations of MC ranging from 0.1 to 10 µM did not cause NADPH-dependent loss of any of the microsomal parameters assessed (Table 1).
TABLE 1.
Effects of MC and ABT on P450 content, CYP3A protein/activity, and POR protein/activity in mouse hepatic microsomes
Data are expressed as means ± S.D. of determinations from four independent pools of mouse hepatic microsomes. Data were analyzed using a repeated-measures design one-way ANOVA and post hoc Newman–Keuls test.
| Condition | P450 Content | CYP3A Protein | Luc-IPA Activity | POR Protein | Cytochrome c Reduction |
|---|---|---|---|---|---|
| nmol/mg protein | % of control | % of control | % of control | nmol/min per mg protein | |
| No drug | |||||
| − NADPH | 0.853 ± 0.073 | 100.0 ± 29.4 | 100.0 ± 30.4 | 100.0 ± 10.4 | 217.9 ± 13.1 |
| + NADPH | 0.645 ± 0.048 | 92.4 ± 29.1 | 142.5 ± 27.5 | 120.2 ± 18.8 | 216.7 ± 22.7 |
| ABT | |||||
| − NADPH | 0.802 ± 0.047 | 92.2 ± 28.5 | 125.8 ± 50.2 | 119.2 ± 12.0 | 216.5 ± 32.6 |
| + NADPH | 0.233 ± 0.062a,b,c | 79.7 ± 35.9 | 3.8 ± 0.9a,b,c | 124.8 ± 20.8 | 221.6 ± 31.9 |
| MC (0.1 µM) | |||||
| − NADPH | 0.759 ± 0.135 | 73.0 ± 26.2 | 120.9 ± 12.9 | 130.3 ± 27.9 | 201.0 ± 26.9 |
| + NADPH | 0.558 ± 0.179a | 65.8 ± 8.7 | 141.3 ± 29.9 | 118.0 ± 25.1 | 211.2 ± 24.6 |
| MC (1 µM) | |||||
| − NADPH | 0.644 ± 0.072 | 75.4 ± 20.2 | 123.2 ± 33.3 | 128.9 ± 36.4 | 184.3 ± 27.4 |
| + NADPH | 0.699 ± 0.121 | 79.1 ± 12.6 | 152.2 ± 37.3 | 125.2 ± 15.2 | 208.1 ± 25.7 |
| MC (5 µM) | |||||
| − NADPH | 0.786 ± 0.144 | 76.6 ± 16.6 | 134.1 ± 32.0 | 105.5 ± 33.9 | 185.7 ± 22.1 |
| + NADPH | 0.650 ± 0.168 | 102.1 ± 20.9 | 161.6 ± 52.1a | 108.2 ± 26.7 | 217.9 ± 35.2 |
| MC (10 µM) | |||||
| − NADPH | 0.743 ± 0.111 | 92.9 ± 20.1 | 128.6 ± 35.8 | 97.3 ± 44.0 | 185.2 ± 34.4 |
| + NADPH | 0.591 ± 0.121 | 91.9 ± 10.4 | 156.3 ± 26.0 | 90.6 ± 24.8 | 212.7 ± 27.5 |
Significantly different (P < 0.05) from No drug – NADPH.
Significantly different (P < 0.05) from No drug + NADPH.
Significantly different (P < 0.05) from ABT – NADPH.
The ability of MC to trigger comparable downregulation of CYP3A11 mRNA and CYP3A protein in the livers of both WT and LCN mice, together with the inactivity of MC as a P450 mechanism-based inactivator, suggests that CYP3A suppression by MC occurs via a pretranslational mechanism that does not require hepatic microsomal P450-mediated metabolism. Although we cannot rule out a role for MC metabolism by non-P450 pathways and/or in cell types other than hepatocytes, our findings are consistent with a key role for the parent drug MC in the CYP3A suppression response. This idea is also supported by our recent report that TCDD, an essentially nonmetabolized HAH-type AHR agonist, suppresses hepatic CYP3A11 mRNA levels in WT but not Ahr-null mice (Lee and Riddick, 2012). Our current working hypothesis is that the suppression of mouse hepatic Cyp3a11 by both TCDD and MC involves AHR activation by the parent compounds. Dioxin-responsive elements (DREs) can be viewed as genomic sites for AHR recruitment with potential for transcriptional induction or suppression depending on the gene context (Riddick et al., 2003). A recent phylogenetic analysis showed that 78% of the CYP3 family genes across a range of teleost species contain three or more DREs, some of which have been implicated in TCDD induction responses (Chang et al., 2013). The same study reported that the human CYP3A4 and mouse Cyp3a11 promoters contain a single putative DRE; any regulatory role in response to AHR activation is unknown.
In conclusion, our studies with LCN mice and the inactivity of MC as a P450 mechanism-based inactivator indicate that MC downregulates mouse hepatic CYP3A protein via a pretranslational mechanism that does not require hepatic microsomal P450-dependent activity. It will be important to determine whether human CYP3A4 is also downregulated in response to HAHs and PAHs and if so, the molecular mechanisms involved and the functional impacts on the biotransformation of therapeutic agents and endogenous substances.
Acknowledgments
The authors thank Dr. Hong Wu (Wadsworth Center) for assistance with mouse work and sample processing.
Abbreviations
- ABT
1-aminobenzotriazole
- AHR
aryl hydrocarbon receptor
- ANOVA
analysis of variance
- CPR or POR
NADPH–cytochrome P450 oxidoreductase
- DRE
dioxin-responsive element
- FMO
flavin-containing monooxygenase
- HAH
halogenated aromatic hydrocarbon
- HRN
hepatic P450 reductase-null
- LCN
liver Cpr-null
- Luc-IPA
luciferin isopropyl acetal
- MC
3-methylcholanthrene
- P450
cytochrome P450
- PAH
polycyclic aromatic hydrocarbon
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- WT
wild-type
Authorship Contributions
Participated in research design: Lee, Ding, Riddick.
Conducted experiments: Lee.
Performed data analysis: Lee, Riddick.
Wrote or contributed to the writing of the manuscript: Lee, Ding, Riddick.
Footnotes
This work was supported by the Canadian Institutes of Health Research [Grant MOP-93759]; and by the National Institutes of Health National Cancer Institute [Grant R01-CA092596].
References
- Cali JJ, Ma D, Wood MG, Meisenheimer PL, Klaubert DH. (2012) Bioluminescent assays for ADME evaluation: dialing in CYP selectivity with luminogenic substrates. Expert Opin Drug Metab Toxicol 8:1115–1130 [DOI] [PubMed] [Google Scholar]
- Celius T, Pansoy A, Matthews J, Okey AB, Henderson MC, Krueger SK, Williams DE. (2010) Flavin-containing monooxygenase-3: induction by 3-methylcholanthrene and complex regulation by xenobiotic chemicals in hepatoma cells and mouse liver. Toxicol Appl Pharmacol 247:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CT, Chung HY, Su HT, Tseng HP, Tzou WS, Hu CH. (2013) Regulation of zebrafish CYP3A65 transcription by AHR2. Toxicol Appl Pharmacol 270:174–184 [DOI] [PubMed] [Google Scholar]
- Finn RD, Henderson CJ, Scott CL, Wolf CR. (2009) Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J 417:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Weng Y, Zhang QY, Cui HD, Behr M, Wu L, Yang WZ, Zhang L, Ding X. (2003) Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J Biol Chem 278:25895–25901 [DOI] [PubMed] [Google Scholar]
- He P, Court MH, Greenblatt DJ, von Moltke LL. (2006) Factors influencing midazolam hydroxylation activity in human liver microsomes. Drug Metab Dispos 34:1198–1207 [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Otto DME, Carrie D, Magnuson MA, McLaren AW, Rosewell I, Wolf CR. (2003) Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem 278:13480–13486 [DOI] [PubMed] [Google Scholar]
- Jenkins RE, Kitteringham NR, Hunter CL, Webb S, Hunt TJ, Elsby R, Watson RB, Williams D, Pennington SR, Park BK. (2006) Relative and absolute quantitative expression profiling of cytochromes P450 using isotope-coded affinity tags. Proteomics 6:1934–1947 [DOI] [PubMed] [Google Scholar]
- Jokinen MJ, Olkkola KT, Ahonen J, Neuvonen PJ. (2001) Effect of rifampin and tobacco smoking on the pharmacokinetics of ropivacaine. Clin Pharmacol Ther 70:344–350 [PubMed] [Google Scholar]
- Kassai A, Toth G, Eichelbaum M, Klotz U. (1988) No evidence of a genetic polymorphism in the oxidative metabolism of midazolam. Clin Pharmacokinet 15:319–325 [DOI] [PubMed] [Google Scholar]
- Katoh M, Watanabe M, Tabata T, Sato Y, Nakajima M, Nishimura M, Naito S, Tateno C, Iwasaki K, Yoshizato K, et al. (2005) In vivo induction of human cytochrome P450 3A4 by rifabutin in chimeric mice with humanized liver. Xenobiotica 35:863–875 [DOI] [PubMed] [Google Scholar]
- Lee C, Ding X, Riddick DS. (2013) The role of cytochrome P450-dependent metabolism in the regulation of mouse hepatic growth hormone signaling components and target genes by 3-methylcholanthrene. Drug Metab Dispos 41:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Hutson JR, Tzau VKF, Riddick DS. (2006) Regulation of constitutive mouse hepatic cytochromes P450 and growth hormone signaling components by 3-methylcholanthrene. Drug Metab Dispos 34:1530–1538 [DOI] [PubMed] [Google Scholar]
- Lee C, Riddick DS. (2012) Aryl hydrocarbon receptor-dependence of dioxin’s effects on constitutive mouse hepatic cytochromes P450 and growth hormone signaling components. Can J Physiol Pharmacol 90:1354–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Montellano PR, Mathews JM. (1981) Autocatalytic alkylation of the cytochrome P-450 prosthetic haem group by 1-aminobenzotriazole. Isolation of an NN-bridged benzyne-protoporphyrin IX adduct. Biochem J 195:761–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovando BJ, Vezina CM, McGarrigle BP, Olson JR. (2006) Hepatic gene downregulation following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 94:428–438 [DOI] [PubMed] [Google Scholar]
- Richert L, Tuschl G, Abadie C, Blanchard N, Pekthong D, Mantion G, Weber JC, Mueller SO. (2009) Use of mRNA expression to detect the induction of drug metabolising enzymes in rat and human hepatocytes. Toxicol Appl Pharmacol 235:86–96 [DOI] [PubMed] [Google Scholar]
- Riddick DS, Lee C, Bhathena A, Timsit YE. (2003) The 2001 Veylien Henderson Award of the Society of Toxicology of Canada. Positive and negative transcriptional regulation of cytochromes P450 by polycyclic aromatic hydrocarbons. Can J Physiol Pharmacol 81:59–77 [DOI] [PubMed] [Google Scholar]
- Roncoroni C, Rizzi N, Brunialti E, Cali JJ, Klaubert DH, Maggi A, Ciana P. (2012) Molecular imaging of cytochrome P450 activity in mice. Pharmacol Res 65:531–536 [DOI] [PubMed] [Google Scholar]
- Shimada T, Guengerich FP. (2006) Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol 19:288–294 [DOI] [PubMed] [Google Scholar]
- Tunek A, Schelin C, Jergil B. (1979) Microsomal target proteins of metabolically activated aromatic hydrocarbons. Chem Biol Interact 27:133–144 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Chamberlain M, Vassieva O, Henderson CJ, Wolf CR. (2005) Relationship between hepatic phenotype and changes in gene expression in cytochrome P450 reductase (Por) null mice. Biochem J 388:857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y, DiRusso CC, Reilly AA, Black PN, Ding X. (2005) Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. J Biol Chem 280:31686–31698 [DOI] [PubMed] [Google Scholar]
- Wood AW, Chang RL, Levin W, Thomas PE, Ryan D, Stoming TA, Thakker DR, Jerina DM, Conney AH. (1978) Metabolic activation of 3-methylcholanthrene and its metabolites to products mutagenic to bacterial and mammalian cells. Cancer Res 38:3398–3404 [PubMed] [Google Scholar]
- Yanagimoto T, Itoh S, Sawada M, Kamataki T. (1997) Mouse cytochrome P450 (Cyp3a11): predominant expression in liver and capacity to activate aflatoxin B1. Arch Biochem Biophys 340:215–218 [DOI] [PubMed] [Google Scholar]
- Zhang J, Chaluvadi MR, Reddy R, Motika MS, Richardson TA, Cashman JR, Morgan ET. (2009) Hepatic flavin-containing monooxygenase gene regulation in different mouse inflammation models. Drug Metab Dispos 37:462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]