Fig. 4.
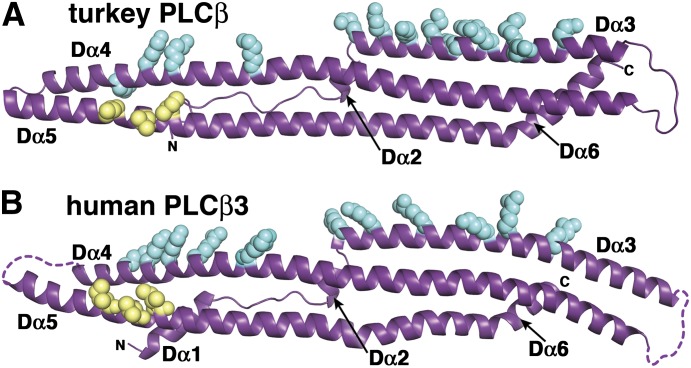
The structure and surface of the distal CTD are conserved. The isolated turkey distal CTD (PDB ID 1JAD) (A) and human PLCβ3 distal CTD (PDB ID 4GNK) (B) have the same fold and similar conserved surfaces. Basic residues within Dα3 and Dα4 (blue spheres) form an extended conserved surface along one face of the domain, which likely functions as a membrane binding site. The conserved hydrophobic patch on Dα5, which interacts with the N-terminal helix of Gαq in the 4GNK structure (Fig. 2), is shown as yellow spheres. The turkey PLCβ CTD was engineered to facilitate crystallization by deletion of 32 residues from the Dα3–Dα4 loop.