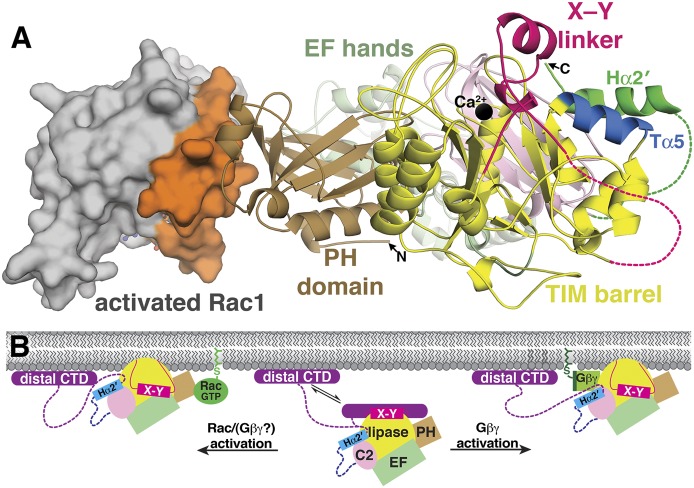
Fig. 6.
Gβγ and Rac1 bind the PLCβ catalytic core. (A) Rac1 (gray surface) binds exclusively to the PH domain via its switch regions (orange surface), which enables PLCβ to detect the activation state of Rac1. PLCβ domains are colored as in Fig. 1. Current biochemical data predict that Gβγ binds to either the PH domain or a helix within the Y domain of the TIM barrel (Tα5, in blue), which is in close proximity to the X–Y linker and the Hα2′ helix. The active site Ca2+ is shown as a black sphere, disordered regions as dashed lines, and GTPγS bound to Rac1 as ball and sticks. (B) Rac1/Gβγ likely share similar PLCβ activation mechanisms. In the resting state (center), PLCβ is in an autoinhibited state, as described in Fig. 5B. Rac1/Gβγ binding to the PLCβ catalytic core is dictated in part by the geometry imposed by the cell membrane, which likely increases the affinity between these activators and PLCβ. The interaction between Rac1/Gβγ and the PH domain (left) or between Gβγ and the catalytic core (right) likely optimize the orientation of the active site at the membrane surface, overcoming repulsion between the membrane and the acidic region in the X–Y linker, and thereby opening access to the active site.