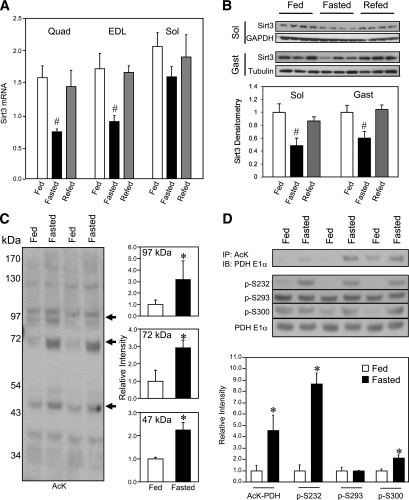
FIG. 1.
Skeletal muscle Sirt3 expression and mitochondrial protein acetylation are regulated by fasting. Wild-type 8-week-old male C57Bl/6 mice were fed ad libitum, fasted for 24 h, or fasted and then refed for 16 h. After each treatment, RNA and protein were extracted and analyzed by real-time quantitative PCR (A) or Western blotting (B) for assessment of Sirt3 expression in quadriceps (Quad), EDL, soleus (Sol), and gastrocnemius (Gast) muscle groups (n = 3–5, #P < 0.05 vs. fed, ANOVA). C: Mixed hindlimb muscles (gastrocnemius and soleus) were collected from mice in the fed or fasted state. Muscle mitochondria were isolated in the presence of protease and deacetylase inhibitors as described in research design and methods. Mitochondrial protein lysates from each animal were subjected to SDS-PAGE and Western blotting using an antibody against AcK. The intensity of specified bands was quantified with ImageJ software (n = 3, *P < 0.05, Student t test). D: PDH E1α acetylation in muscle of fed or fasted mice was measured by immunoprecipitation (IP) of mitochondrial lysates using anti-AcK antibody. Immunoprecipitates were subjected to Western blotting analysis (IB) using anti–PDH E1α antibody. The same muscle mitochondrial lysates were directly subjected to SDS-PAGE electrophoresis and Western blotting using antibodies against phosphorylated serine sites p-232, p-293, and p-300 of the PDH E1α subunit and total protein of PDH E1α. Autoradiography of Western blots was quantified with ImageJ software (n = 3, *P < 0.05, Student t test). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.