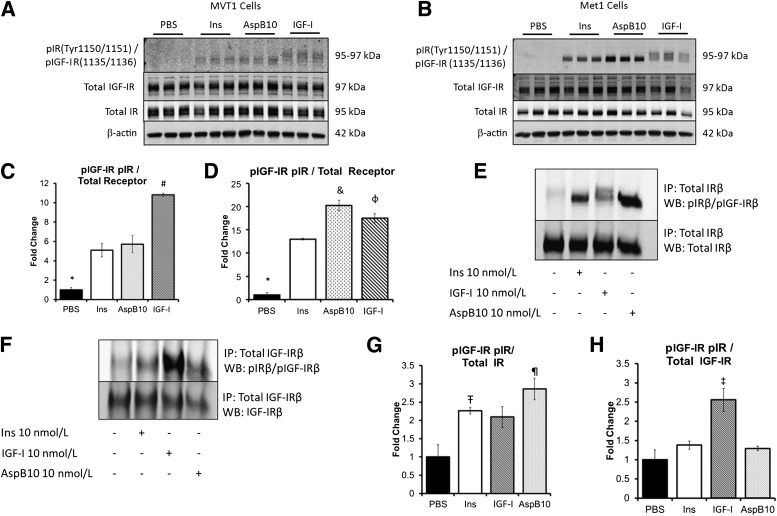
FIG. 1.
Insulin and AspB10 led to IR phosphorylation but not IGF-IR phosphorylation in vitro. MVT1 and Met1 cells were stimulated with 10 nmol/L insulin (Ins), the insulin analog AspB10 (AspB10), rhIGF-I (IGF-I), or PBS. Western blot (WB) analysis of protein lysates (from MVT1 cells, A; from Met1 cells, B) and densitometry (from MVT1 cells, C; and from Met1 cells, D) demonstrated that insulin and AspB10 led to phosphorylation of the IRβ(Tyr 1150/1151) at 95 kDa, whereas IGF-I stimulation led to phosphorylation of the IGF-IRβ(Tyr 1135/1136) at 97 kDa and the IRβ(Tyr 1150/1151) at 95 kDa, most likely through hybrid receptor phosphorylation (A and B). Immunoprecipitation (IP) of the IR in Met1 cell lysates after in vitro stimulation with PBS, 10 nmol/L Ins, IGF-I, or AspB10 confirmed that insulin and AspB10 caused phosphorylation of the IRβ(Tyr 1150/1151), whereas IGF-I led to IGF-IRβ(Tyr 1135/1136) and IRβ(Tyr 1150/1151) receptor phosphorylation, the latter likely through IR and hybrid receptor (E and F) with densitometry (G and H). Representative blots from experiments are shown. All experiments were performed two to three times. Bar graphs display means and SEM. *PBS group significantly lower than all other groups, P < 0.05; #IGF-I group significantly higher than all other groups, P < 0.05; &AspB10 group significantly higher than human insulin group, P < 0.05; ΦIGF-I group significantly higher than human insulin group, P < 0.05; Ŧhuman insulin group significantly greater than PBS, P < 0.05; ¶AspB10 significantly greater than PBS, P < 0.05; ‡IGF-I group greater than PBS, P = 0.05. p, phosphorylated.