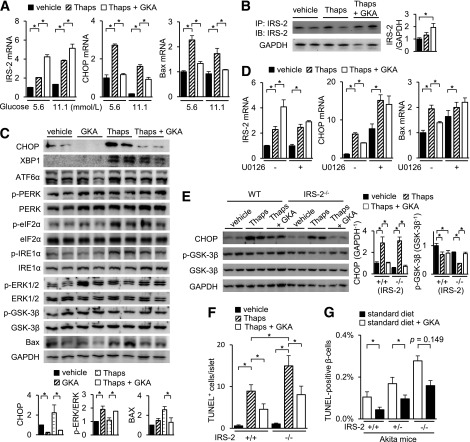
FIG. 6.
GKA ameliorates ER stress–induced apoptosis via IRS-2–independent pathway. Islets from C57BL/6J mice (aged 8 weeks) were incubated with 1 μmol/L thapsigargin (Thaps) and 30 μmol/L GKA (Cpd A) at 5.6 (A–C) or 11.1 mmol/L (A) glucose for 24 h. A: mRNA expression levels in the islets (n = 6). B, left: The immunoprecipitated or total cell extracts from the islets were subjected to immunoblotting as indicated. B, right: Intensity of the signals quantified by densitometry (n = 4). C, top: The total cell extracts from the islets were subjected to immunoblotting as indicated. C, bottom: Intensity of the signals quantified by densitometry (n = 4). D: Islets of C57BL/6J mice (aged 8 weeks) were incubated with 30 μmol/L GKA or 1 μmol/L Thaps at 5.6 mmol/L glucose for 24 h in the presence of 20 μmol/L U0126 (MEK inhibitor). The mRNA expression levels (n = 6). E and F: Islets of WT (IRS-2+/+) or IRS-2−/− mice were incubated with 1 μmol/L Thaps and 30 μmol/L GKA for 24 h at 5.6 mmol/L glucose. E: mRNA expression levels in the islets (n = 6). F: Number of TUNEL-positive β-cells in the islets (at least 30 islets per indicated group). G: IRS-2+/+;Akita, IRS-2+/−;Akita, or IRS-2−/−;Akita mice were fed standard diet containing GKA from day 19 to 33 after birth. The proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the pancreas sections (aged 33 days, n = 4–7). *P < 0.05. IB, immunoblotting; IP, immunoprecipitation.