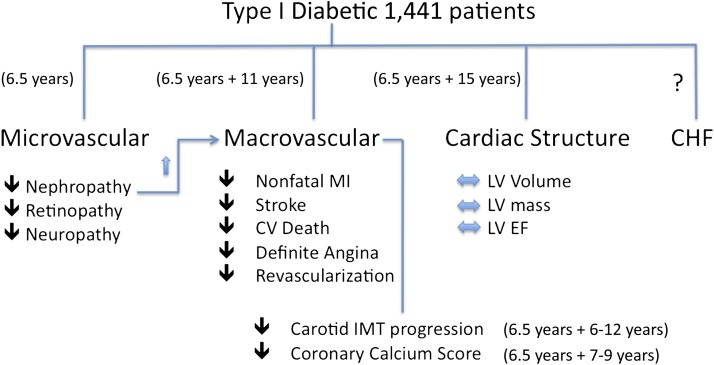
FIG. 1.
Overview of the various cardiovascular and related end points from the DCCT and the EDIC study. Arrows indicate the direction of change in frequency of each complication (increased, decreased, or no change) in the DCCT group receiving intensive treatment relative to the conventional treatment group during the 6.5 years of the DCCT. The treatment during DCCT + duration of EDIC follow-up is indicated for each category. The presence of proteinuria significantly increased the incidence of cardiovascular events. Heart failure events have not been reported in these trials. CV, cardiovascular; EF, ejection fraction; IMT, intima-media thickness; MI, myocardial infarction.