Abstract
OBJECTIVE
To determine whether type A behavior predicts all-cause mortality and incident coronary artery disease (CAD) in a type 1 diabetic population.
RESEARCH DESIGN AND METHODS
Follow-up data (22 years) from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study of childhood-onset type 1 diabetes were analyzed for the 506 participants who completed the Bortner Rating Scale (measuring type A behavior) and Beck Depression Inventory (BDI) at baseline (1986–1988). CAD comprised myocardial infarction as determined by hospital records/Q waves on electrocardiogram (ECG), CAD death (determined by a mortality classification committee), angiographic stenosis, ischemic ECG, and angina.
RESULTS
There were 128 deaths (25.3%) during follow-up. Univariate analysis showed an inverse relationship between Bortner scores and all-cause mortality (P = 0.01), which remained significant after allowing for age, sex, duration, HbA1c, education, smoking, BMI, and physical activity (P = 0.03). However, the addition of BDI scores attenuated the relationship (P = 0.11) with a significant interaction (P = 0.03) such that any protective effect against mortality was limited among individuals with lower BDI scores (bottom three quintiles) (P = 0.07), whereas no effect was seen in those with higher BDI scores (P = 0.97). Bortner scores showed only a borderline association with incident CAD (P = 0.09).
CONCLUSIONS
Those with higher type A behavior have lower all-cause mortality in our type 1 diabetic population, an effect that interacts with depressive symptomatology such that it is only operative in those with low BDI scores. Further research should focus on understanding this interaction.
The incidence of type 1 diabetes, which remains incurable, has continued to rise annually by ∼3% (1). Unfortunately, prevention is not currently feasible. Therefore, the exploration of type 1 diabetes complications, untimely mortality and the associated risk factors, must continue. Type A behavior has been described as an action-emotion complex, meaning that the behavior is elicited by the outside environment (2). People characterized as having type A behavior tend to focus toward achieving and accomplishing more in less time than others. Because of these tendencies, these people tend to be competitive, aggressive, time urgent, and work oriented and can become annoyed if things are not achieved in a time frame they find sufficient (2). Therefore, it seems that type A behaviors are not a set of personality characteristics that come about due to the environment; rather, the behavior is a result of predispositions within a person that are exhibited due to specific situations (2). In an earlier review, Matthews and Haynes (2) noted that although type A behavior was linked to increased coronary heart disease (CHD) risk in the general population, findings were consistently negative in high-risk populations. For example, the prospective Western Collaborative Group Study (WCGS) found that those with type A behavior experienced an increased rate of CHD compared with type B behavior (P = 0.001) (3). However, in their high-risk population who had already undergone a CHD event, type A behavior had a lower CHD-associated mortality rate in those surviving 24 h or more than those characterized as having type B behavior (P = 0.03) (4). Therefore, it appears that type A behavior may have different effects on health depending on underlying chronic disease status.
Little is known about the psychosocial contribution to the increased coronary artery disease (CAD) risk seen in people with type 1 diabetes beyond depression (5,6), in particular, whether type 1 diabetes is an additional high-risk group in which the inverse association between type A behavior and CAD/mortality exists. Cross-sectional data from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study have shown (using the Bortner Rating Scale) that participants with multiple complications, including CAD, retinopathy, neuropathy, and/or nephropathy, reported less type A behaviors than those without complications (P < 0.05) (5). The long length of follow-up now available in the EDC allowed a prospective analysis of the role of type A behavior in mortality and CAD, and we are unaware of other investigations of this relationship. We also investigated the association between type A behavior and CAD-related mortality in those already diagnosed with CAD and whether the established effect of depressive symptomatology on CAD incidence interacted with or explained any effect of type A behavior. Thus, the aims of the current study were to investigate the relationships between type A behavior and mortality, type A behavior and incident CAD, and type A behavior and mortality among those with CAD during 22 years of follow-up.
RESEARCH DESIGN AND METHODS
The EDC study is comprised of participants diagnosed with type 1 diabetes between 1950 and 1980 at <17 years of age, seen within 1 year of diagnosis at the Children’s Hospital of Pittsburgh. Biennial follow-up occurred since baseline in 1986–1988, which included questionnaires with physician examinations and laboratory analyses of urine and blood for the first 10 years and again at 18 years. Data up to the 22-year follow-up are now available. Participants ≥18 years of age at study entry completed the Bortner Rating Scale, which measures aspects of type A behavior (7) and has been shown to have good test-retest reliability (2). Participants were asked to circle the dot on the line that represents where they believed they fell between two different sentences. Some examples of the sentences included “never late” versus “casual about appointments,” “always rushed” versus “never rushed, even under pressure,” and “take things one at a time” versus “try to do many things at once, thinking about what I am going to do next.” CAD was defined as myocardial infarction confirmed by hospital records or Q waves on electrocardiogram (Minnesota Code 1.1 or 1.2); coronary artery stenosis, defined as ≥50% blockage, or revascularization; ischemic electrocardiogram, defined using Minnesota Code 1.3, 4.1–4.3, 5.1–5.3, or 7.1; angina, diagnosed by an EDC physician; or CAD death (determined by a mortality classification committee).
Overall mortality, including CAD-associated mortality and complication status, was determined as of 25 February 2011. Searches were performed in both the Social Security Death Index and the National Death Index. In order to confirm each death, death certificates were obtained, plus, as appropriate, 1) hospital records, 2) autopsy/coroner reports, and 3) interview with next of kin regarding the death. The underlying causes of death, and the hierarchal order for all contributing causes of death, were determined by a mortality classification committee consisting of two or more physician epidemiologists. This method is based on standardized procedures (8).
The following covariates were chosen as potential predictors for our final model: age, sex, duration, education, physical activity, smoking, BMI, insulin dosage, HbA1c, and depressive symptomatology. These covariates were chosen because they are previously demonstrated risk factors for CAD and/or early mortality in type 1 diabetes (9). Education was assessed using a five-point scale, i.e., some high school, high school graduate, some college, bachelor’s degree, and graduate education beyond bachelor’s. Physical activity was assessed using questions about current levels of leisure activities (10), as well as by estimating the energy expenditure over the past week (kcal/week) through use of questions asking about the daily number of flights of stairs climbed, the number of blocks walked daily, and all sports participation that had occurred over the past week. Ever smoked was defined as having had >100 cigarettes over their lifetime. Insulin dosage was expressed as the number of units of insulin used per day divided by the participant’s weight in kilograms. BMI was calculated as participant’s weight in kilograms divided by the square of their height in meters. Fasting blood samples were analyzed for HbA1 (microcolumn cation exchange; Isolab, Akron, OH), and these original HbA1 values were converted to Diabetes Complications and Control Trial (DCCT)–aligned HbA1c for all analyses using a regression equation derived from duplicate assays (DCCT HbA1c = 0.14 + 0.83[EDC HbA1]). Finally, depressive symptomatology was measured using the Beck Depression Inventory (BDI) (11). The BDI is a 21-item self-report scale that is widely used in both healthy and ill populations. A score of 0–9 indicates minimal depression, 10–18 indicates mild depression, 19–29 indicates moderate depression, and 30–63 indicates severe depression (11). BDI scores have been shown to approximate clinically significant symptoms of depression (11).
Cox proportional hazards models were used to examine the univariate and multivariable relationship between baseline Bortner scores and overall mortality, CAD incidence over 22 years of follow-up, and CAD-related mortality among those with CAD. To assess univariate associations between baseline Bortner score and potential covariates (i.e., age, sex, duration, education, physical activity, smoking, BMI, insulin dosage, HbA1c, and depressive symptomatology), Student t test or Wilcoxon signed rank test was used as appropriate. Cox proportional hazards modeling was used to examine the independent association between Bortner score and each outcome (overall mortality, CAD incidence, and incident CAD death among those with CAD), adjusting for significant baseline covariates. All statistics were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
At the EDC baseline exam, 658 participants were seen. One hundred and fifty two participants were excluded from this analysis for having missing covariate measures; however, 60 of these participants were <18 years of age and therefore not eligible to complete the Bortner or the BDI, and an additional 92 participants were excluded, most commonly, for missing data on the BDI, Bortner, or physical activity measures.
As of 25 February 2011, of EDC participants who completed both the Bortner and the BDI at baseline, and who had complete covariate data (n = 506, 250 males and 256 females), there were 128 deaths (25.3%). Those excluded were less likely to have a high school education (P = 0.01) and were more likely to be smokers (P < 0.01), but did not differ significantly for age, duration, sex, HbA1c, physical activity, BMI, or depressive symptomatology (Supplementary Table 1).
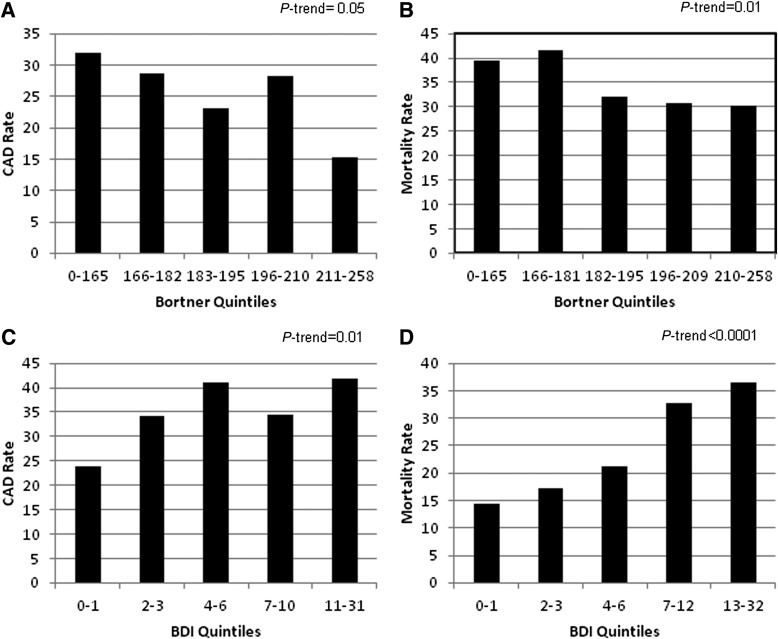
Significant covariate differences existed between those with and without incident CAD for age, duration, physical activity, smoking, BDI, and insulin dosage (Table 1). A significant trend was demonstrated for both Bortner (P = 0.05) and BDI scores (P = 0.01) at baseline and CAD incidence (Fig. 1A and C). A borderline univariate relationship was seen between baseline Bortner scores and CAD incidence (P = 0.09). No significant interaction was observed between Bortner and BDI in relation to CAD incidence.
Table 1.
Baseline characteristics by CAD incidence, 1986–1988
Figure 1.
A: Bortner quintiles and CAD rate. B: Bortner quintiles and mortality rate. C: BDI quintiles and CAD rate. D: BDI quintiles and mortality rate.
Differences existed by subsequent mortality for most baseline covariates. Deceased participants tended to be older, with longer diabetes duration, male, less physically active, and ever smokers and had a higher HbA1c and BDI score and a lower Bortner score compared with survivors (Table 2). The univariate association between Bortner scores and all-cause mortality is shown in more detail by quintiles (P = 0.01) (Fig. 1B). Those with higher type A behavior tended to be at a reduced risk for mortality with a significant trend (P = 0.01). Multivariable analyses (Table 3) of the association between type A behavior and all-cause mortality were performed with four models, progressively controlling for covariates. Model 1 was adjusted for age and sex, with Bortner score remaining significant (P = 0.01). Model 2 included age, sex, duration, HbA1c, education, smoking, BMI, and physical activity as covariates and demonstrated that Bortner score continued to significantly predict mortality (hazard ratio [HR] 0.99 [95% CI 0.98–1.00]; P = 0.03). For every one-point increase on the Bortner scale, there was a 1% lower mortality risk (Bortner Rating Scale range, 97–258). However, further adjustment for BDI (model 3) reduced the association between Bortner and mortality (P = 0.11).
Table 2.
Baseline characteristics (1986–1988) by subsequent mortality
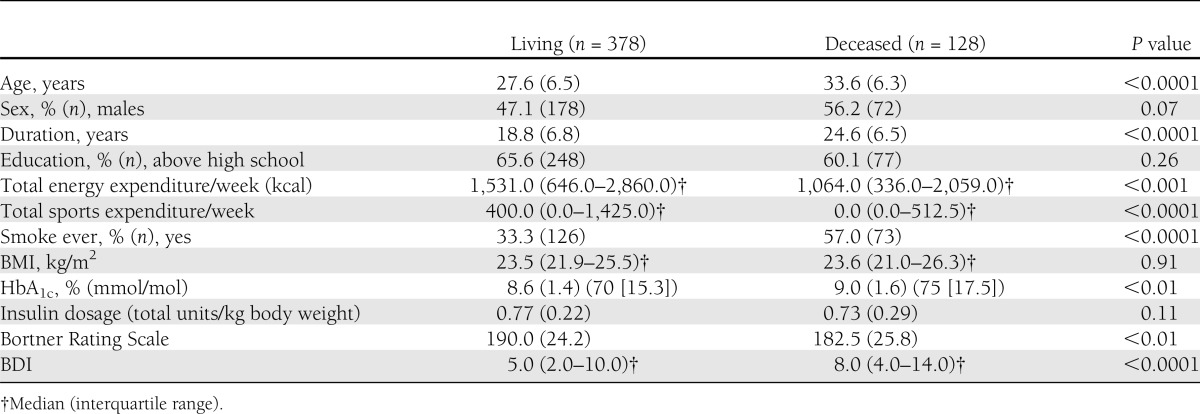
Table 3.
Association between type A behavior and all-cause mortality in type 1 diabetes
Model 4 tested for an interaction between the Bortner Rating Scale and BDI for mortality (Table 3) as a significant inverse correlation was found between the two (r = −0.18, P < 0.001). This interaction was significant (P = 0.03), meaning type A behavior is only operative in those with lower BDI scores. Further BDI-stratified analyses were then performed. Based on the quintiles determined in our study population (BDI scores: 0–1, 2–3, 4–6, 7–12, and 13–32) (Fig. 1D), we compared the first three quintiles with the upper two quintiles, resulting in two categories, a BDI score ≤6 versus a BDI score ≥7. A borderline significant protective effect against mortality was seen with higher type A behavior score in the lower BDI quintiles (P = 0.07), but not with a BDI score ≥7 (P = 0.97).
Performing analyses by sex (mean Bortner score in men, 191.1, vs. women, 185.2; P < 0.01), a significant univariate relationship between Bortner and mortality (0.98 [0.97–0.99]; P < 0.001) was seen in men but not in women (0.99 [0.98–1.00]; P = 0.12). However, a greater proportion of men than women were type A within our population (P = 0.03). The significant relationship among men remained after multivariable adjustment (0.99 [0.98–0.99]; P = 0.03) but was attenuated after further adjusting for BDI (0.99 [0.98–1.00]; P = 0.10). A significant interaction between Bortner and BDI (P = 0.03) was noted, although stratification by the same cut points of BDI as above did not yield any significant differences. Stratifying by minimal to mild versus moderate to severe depressive symptoms, however, demonstrated that men with a minimal to mild BDI score (0.98 [0.97–0.99]; P = 0.02) were protected against mortality compared with those with a moderate to severe BDI score (1.00 [0.97–1.04]; P = 0.63) in multivariable analyses.
Out of 506 participants, there were 64 CAD-related deaths (12.6%). We found that Bortner significantly predicted CAD mortality (0.99 [0.98–1.00]; P = 0.04). The analyses were subsequently repeated excluding non-CAD deaths from the control group (essentially comparing survivors to CAD death [14.3%]), and a significant relationship between Bortner score and CAD death was found as well (0.98 [0.97–0.99]; P = 0.03).
We then examined the predictive value of the Bortner Rating Scale for CAD mortality in those with prevalent CAD. No univariate association was found with death among those with CAD within 22 years of follow-up (P = 0.35).
CONCLUSIONS
We observed a significant relationship between Bortner scores and all-cause mortality, which was attenuated after adjustment for BDI. We also noted the presence of significant effect modification of the relationship between the Bortner and mortality by BDI score. Thus, a borderline significant inverse association between type A behavior and mortality was only apparent among those in the bottom three BDI quintiles, whereas this relationship was lost in the top two quintiles. Analyses stratifying by sex suggested that only men were protected against mortality with a higher type A behavior score, even after adjustment for BDI. However, stratifying by BDI revealed a protective effect of type A behavior only in those with minimal to mild, but not moderate to severe, depressive symptoms. We found a borderline significant relationship between Bortner scores and incident CAD, which was attenuated after adjustment for duration.
To our knowledge, this is the first study to investigate the relationship between type A behavior and all-cause mortality in a type 1 diabetic population. The strengths of our study are the long follow-up and the completeness of data obtained for our population. In addition to demonstrating the importance of type A behavior and depressive symptomatology, our results affirm the role traditional, important covariates play on CAD development and early mortality in type 1 diabetes. Those with the highest type A behavior scores were at the lowest mortality risk, which is consistent with most of the literature demonstrating that high-risk populations are protected with greater type A behavior (2,3,5). However, with the addition of BDI to our model, this relationship was attenuated. After determining that the Bortner scale and BDI were inversely correlated, we tested for an interaction between them to determine if the protective effect we observed from high type A behavior was really due to the low depressive symptomatology score in this group. The interaction term was significant, suggesting that type A behavior may be protective against mortality in the absence of depressive symptomatology (although this was only borderline significant). Any protection from type A behavior appears to be lost once the higher quintiles of depressive symptomatology are reached. This suggests that depressive symptomatology is a stronger predictor of mortality than type A behavior in type 1 diabetes. Indeed, the death rate was 17.8% in the bottom three quintiles, approximately two times lower than in the top two quintiles, at 34.5%.
The importance of depressive symptomatology in type 1 diabetes is expected, as it has been frequently demonstrated that those with high depressive symptomatology are at an increased mortality (12) and morbidity risk (including diabetes complications) (13). Comorbid depression and type 1 diabetes is also associated with poorer diabetes self-management and metabolic control, decreased quality of life, and higher health care usage (12). Our previous research showed that BDI score significantly predicted CHD even after controlling for hypertension, waist-to-hip ratio (WHR), white blood cell count, fibrinogen, smoking status, distal symmetric polyneuropathy, and overt nephropathy. However, this relationship became attenuated after the addition of all possible variables in the mediation analysis (6). Depressive symptomatology has also been found to increase WHR in both sexes (14) and appears to play an important role in the incidence and progression of type 1 diabetes–associated complications, as confirmed in our study.
We hypothesized that Bortner scores would continue to be predictive of mortality, even after controlling for BDI, particularly because individuals with diabetes have to adopt regimented control along with other characteristic type A behaviors, such as having to do many things at once, thinking of what they might need to do next, becoming less casual about things, and feeling ambitious (7). Type A behaviors may increase the efficiency in which an individual cares for their type 1 diabetes, therefore preventing complications and early mortality. However, depressive symptoms may outweigh the significance of type A behavior, as demonstrated in our analysis. Adopting type A behaviors in order to better care for a chronic disease like type 1 diabetes may also partially explain why type A behavior is protective in high-risk, as opposed to the general, populations. Those with greater type A behavior in the presence of a chronic disease may treat symptoms and suspected complications more seriously and intensively than those characterized as having less type A behavior.
Little research exists on depressive symptomatology and subsequent risk of mortality in type 1 diabetes. The FinnDiane Study Group concluded that in women, baseline antidepressant agent purchase (their surrogate marker for depression) was associated with an increased mortality risk over 9 years of follow-up (2.15 [1.34–3.45]) (15). Although this association was only seen in women, our results demonstrate a similar relationship. Those with increased depressive symptomatology were not only at increased mortality risk, but the protection offered by type A behavior disappeared with increased BDI scores. Depressive symptoms, therefore, appear to play a very important role in predicting mortality in type 1 diabetes.
Investigating the association between type A behavior and mortality by sex showed that the protective effects of type A behavior are only significant in men. However, these findings may be partially attributable to a lack of power to detect the relationship in women as fewer women had a high type A score. The relationship among men remained until BDI adjustment. We compared those with minimal to mild versus moderate to severe depressive symptoms and found only those with high type A behavior and less than moderate depressive symptoms were protected against mortality.
It has been previously noted that because type A behavior questionnaires can be interpreted as geared toward work or competitive behaviors, men may respond differently than women (2). In other words, men may feel it is more socially acceptable, expected, and fitting of their traditional role to declare themselves as “very competitive,” “hard driving,” and “ambitious,” whereas women may not feel the pressure to fulfill that stereotype. Therefore, our male participants’ responses on the Bortner scale may differ compared with women due to social norms, especially in the 1980s when the questionnaire was completed. At that time, if women were homemakers, perhaps they felt they were not facing the daily demands of a career and therefore had less type A responses. This is consistent with our data, as we saw a statistically higher mean type A behavior score in men compared with women (191.1 vs. 185.2; P < 0.01). A study that also administered the Bortner scale in the 1980s found that participants with no or minimal obstructive CAD had higher type A scores compared with those with obstructive disease. After further analysis by sex, the effect was only significant in men, consistent with our findings (16).
We also examined the relationship between Bortner scores and CAD as it is a major contributor to death. In those free of CAD at baseline, type A behavior predicted CAD during 22 years of follow-up, although this was of borderline statistical significance, and this relationship was attenuated after adjustment for duration. Thus, we did not find that in our population, increased type A behavior was protective against CAD or indeed CAD death among those with CAD. Other factors not measured in our study may play a role, and further research is needed to determine which other covariates may offer protection against CAD. Our results were not as hypothesized; however, it should be noted that type A behavior was also not detrimental to the development of CAD, which supports previous research in other high-risk groups (2,3). Because type A behavior was not related to CAD development, we evaluated whether mortality was predicted by Bortner scores based on whether the primary cause of death was CAD or non-CAD related. We found a significant difference between these two groups, with type A behavior protecting against CAD-related death, and again when comparing CAD death to survivors only.
A previous study by Lloyd et al. (5) concluded that lower type A behavior scores were associated with an increased macrovascular disease risk; however, the current study is the first that we are aware of to demonstrate that type A behavior in type 1 diabetes is protective against CAD death, specifically. In a 10-year follow-up study of middle-aged, employed men, specific personality traits that would be considered type A did not predict CAD death (17). The literature examining the relationship between type A behavior and CAD death is limited. Our remarkable finding that type A behavior is specifically protective against CAD death merits further investigation.
Thus far, study findings examining type A behavior in type 1 diabetes are conflicting and often focused on surrogate outcomes (such as glycemic control and complications) due to short length of follow-up time. The results of studies examining type A behavior and glycemic control were mixed, with some suggesting no association (18–20), others a detrimental association (21,22), and another a protective association of specific type A behaviors (i.e., neuroticism) (23). However, the majority of these studies were conducted three decades ago with large amounts of bias, which may explain why mixed results were demonstrated. The majority of the previously published studies were conducted cross-sectionally, using very small sample sizes, and used univariate methods of analysis only. Those that used multivariable analyses only controlled for a few relevant covariates. Lloyd et al. (14) found that those type 1 diabetic participants with multiple complications reported less type A behavior than those without any complications (P < 0.05) (5). In a separate study, it was also determined that in men, lower type A behavior score was predictive of an increased WHR. Because type A behavior was not shown to be detrimental in type 1 diabetes, and protective against complications as a whole and WHR, our hypothesis was generated that with longer follow-up, higher type A behavior may be protective against mortality. Future research needs to take place to examine this relationship in other high-risk populations.
Based on the literature that high-risk groups are protected by their type A behavior, we investigated the relationship between the Bortner Rating Scale and CAD case-fatality rate. We hypothesized that in this very high-risk group of people with both type 1 diabetes and CAD, type A behavior would be even more protective, but a relationship was not found. This may be due to several reasons, one being that we may have had an insufficient sample size to find a statistically significant result (28 deaths/125 with CAD). Another reason may be that these participants were too unhealthy to benefit from type A behavior at all, being that they have both type 1 diabetes and a serious complication. Another explanation may be that the type A behaviors were initiated at too late a time in life, and that type 1 diabetes and CAD had already done too much physical damage for any protective effect to take place against mortality. Perhaps behavior type is also a trait and can therefore be modified. If so, this has great implications for care as we can support behavior change to improve self-management, improving the health of those living with diabetes.
There were several strengths and limitations of our study. As mentioned previously, our long follow-up time allowed for us to use mortality as our outcome, as opposed to a surrogate end point such as complication status. Additionally, this was the first study to investigate the relationship between type A behavior and mortality in type 1 diabetes, providing data where there currently are none. Limitations of our study include our small sample size for detecting incident CAD death among those with CAD, which may have led to null results. Another limitation is the possibility of residual confounding; however, we feel we included predictors that are essential for investigating mortality in type 1 diabetes. Furthermore, there were up to 22 years of follow-up time between measuring type A behavior and mortality and/or the onset of CAD; however, we attempted to control for this in the analysis through use of Cox proportional hazards models.
In conclusion, future research is needed to investigate the interaction between BDI and type A behavior, as the latter was only protective in those with low depressive symptomatology. Further research is also needed to explore the protective relationship between type A behavior and CAD death. Understanding these relationships is an important next step in exploring the effects of psychosocial factors on mortality in type 1 diabetes.
Acknowledgments
The Pittsburgh EDC study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-034818).
No potential conflicts of interest relevant to this article were reported.
C.E.F. wrote the manuscript and performed the data analysis. C.E.L. reviewed and edited the manuscript. T.C. and R.G.M. contributed to the data analysis and reviewed and edited the manuscript. T.J.O. researched data, contributed to the data analysis, and reviewed and edited the manuscript. All authors read and approved the final version of the manuscript. T.J.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the long-term help of the EDC participants.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0266/-/DC1.
References
- 1.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002;51:3353–3361 [DOI] [PubMed] [Google Scholar]
- 2.Matthews KA, Haynes SG. Type A behavior pattern and coronary disease risk. Update and critical evaluation. Am J Epidemiol 1986;123:923–960 [DOI] [PubMed]
- 3.Rosenman RH, Brand RJ, Sholtz RI, Friedman M. Multivariate prediction of coronary heart disease during 8.5 year follow-up in the Western Collaborative Group Study. The Am J Cardiol 1976;37:903–910 [DOI] [PubMed]
- 4.Ragland DR, Brand RJ. Type A behavior and mortality from coronary heart disease. N Engl J Med 1988;318:65–69 [DOI] [PubMed] [Google Scholar]
- 5.Lloyd CE, Matthews KA, Wing RR, Orchard TJ. Psychosocial factors and complications of IDDM. The Pittsburgh Epidemiology of Diabetes Complications Study. VIII. Diabetes Care 1992;15:166–172 [DOI] [PubMed]
- 6.Kinder LS, Kamarck TW, Baum A, Orchard TJ. Depressive symptomatology and coronary heart disease in type I diabetes mellitus: a study of possible mechanisms. Health Psychol 2002;21:542–552 [DOI] [PubMed]
- 7.Bortner RW. A short rating scale as a potential measure of pattern A behavior. J Chronic Dis 1969;22:87–91 [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Epidemiology Research International Mortality Study Group. International evaluation of cause-specific mortality and IDDM. Diabetes Care 1991;14:55–60 [DOI] [PubMed] [Google Scholar]
- 9.Costacou T, Orchard TJ. Differential effect of glycemia on the incidence of hypertension by sex: the epidemiology of diabetes complications study. Diabetes Care 2013;36:77–83 [DOI] [PMC free article] [PubMed]
- 10.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 1978;108:161–175 [DOI] [PubMed] [Google Scholar]
- 11.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review 1988;8:77–100
- 12.Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract 2010;87:302–312 [DOI] [PubMed]
- 13.Lloyd CE, Roy T, Nouwen A, Chauhan AM. Epidemiology of depression in diabetes: international and cross-cultural issues. J Affect Disord 2012;142(Suppl.):S22–S29 [DOI] [PubMed]
- 14.Lloyd CE, Wing RR, Orchard TJ. Waist to hip ratio and psychosocial factors in adults with insulin-dependent diabetes mellitus: the Pittsburgh Epidemiology of Diabetes Complications study. Metabolism 1996;45:268–272 [DOI] [PubMed] [Google Scholar]
- 15.Ahola AJ, Harjutsalo V, Saraheimo M, Forsblom C, Groop P-H. Purchase of antidepressant agents by patients with type 1 diabetes is associated with increased mortality rates in women but not in men. Diabetologia 2012;55:73–79 [DOI] [PubMed]
- 16.Bass C, Wade C. Type A behaviour: not specifically pathogenic? Lancet 1982;2:1147–1150 [DOI] [PubMed]
- 17.Shekelle RB, Vernon SW, Ostfeld AM. Personality and coronary heart disease. Psychosom Med 1991;53:176–184 [DOI] [PubMed]
- 18.Sensky T, Petty R. Type A behaviour pattern and glycaemic control in type I diabetes. Psychother Psychosom 1989;52:96–100 [DOI] [PubMed] [Google Scholar]
- 19.Stabler B, Morris MA, Litton J, Feinglos MN, Surwit RS. Differential glycemic response to stress in type A and type B individuals with IDDM. Diabetes Care 1986;9:550–552 [DOI] [PubMed]
- 20.Cox DJ, Taylor AG, Nowacek G, Holley-Wilcox P, Pohl SL, Guthrow E. The relationship between psychological stress and insulin-dependent diabetic blood glucose control: preliminary investigations. Health Psychol 1984;3:63–75 [DOI] [PubMed] [Google Scholar]
- 21.Stabler B, Morris MA, Litton J, Feinglos MN, Surwit RS. Differential glycemic response to stress in type A and type B individuals with IDDM. Diabetes Care 1986;9:550–552 [DOI] [PubMed] [Google Scholar]
- 22.Peyrot M, McMurry JF., Jr Psychosocial factors in diabetes control: adjustment of insulin-treated adults. Psychosom Med 1985;47:542–557 [DOI] [PubMed] [Google Scholar]
- 23.Taylor MD, Frier BM, Gold AE, Deary IJ. Psychosocial factors and diabetes-related outcomes following diagnosis of type 1 diabetes in adults: the Edinburgh Prospective Diabetes Study. Diabet Med 2003;20:135–146 [DOI] [PubMed]