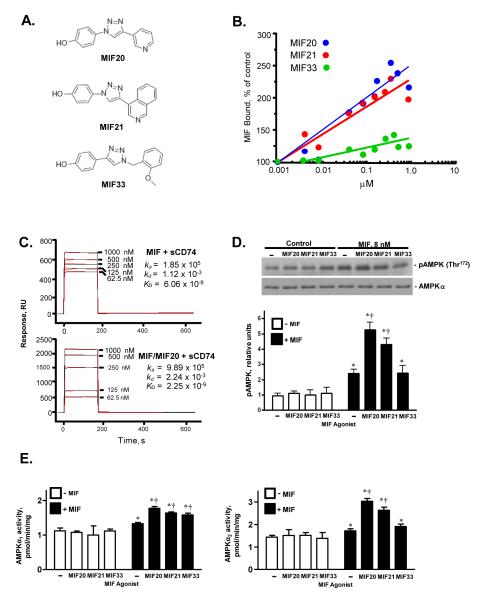
Figure 1.
Small molecule MIF agonists enhance MIF binding to its receptor, CD74, and augment MIF-dependent AMPK phosphorylation. (A) Chemical structures of MIF agonists. MIF20, MIF21, and MIF33 correspond to compounds 5a, 5c, and 3d in ref. 16. (B) Concentration-dependent binding studies of the interaction of biotinylated-MIF (83 nM) with immobilized sCD74 (CD7473-232) in the presence of increasing concentration of MIF agonist. Values shown are the mean of triplicate measurements and are representative of at least two experiments. (C) Measurement of the equilibrium dissociation constants between MIF and sCD74 in the presence of MIF20 by surface plasmon resonance (BIAcore analysis). The MIF receptor ectodomain (sCD74) was immobilized onto CM5 chips and increasing concentrations of MIF introduced into the flow phase together with vehicle (upper panel) or different concentrations of MIF20 (lower panel). (D) MIF-dependent activation of the intracellular phosphorylation of AMPK in mouse cardiomyocytes is enhanced by MIF20 and MIF21. Cultured mouse cardiomyocytes were stimulated with MIF (8 nM) together with test agonists (80 nM) for 20 mins. The cells then were washed, lysed and the intracellular content of pAMPK at Thr172 and AMPKα quantified by western blotting. Densitometric analyses show the mean ± SEM of at least 3 independent experiments. (E) Activation of AMPK complexes containing α1 and α2 isoforms of the catalytic subunit in cardiomyocytes in response to MIF and small molecule MIF agonists assessed by immunoprecipitation and phosphorylation of the target SAMS peptide in vitro. Data are mean + SEM of 3 independent experiments. (*p<0.05 vs. corresponding control, respectively; †p<0.05 vs. MIF alone).