Figure 4.
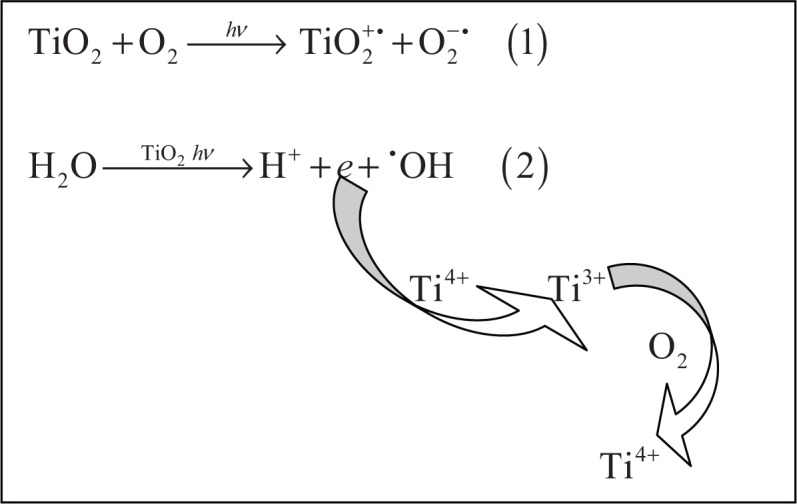
Superoxide anion radical (O2−•), equation (1) and hydroxyl radical (•OH), equation (2) formation resulting from the photo-excitation of TiO2. An electron transfer from photo-excited TiO2 to molecular oxygen leads to production of the superoxide anion radical. Hydroxyl radicals can be formed by electron release from water catalyzed by photo-excited TiO2. By reoxidation of the Ti3+ ions back to Ti4+ ions, the process can start again. Similar generation of superoxide anion and hydroxyl radicals occurs in the case of ZnO.
