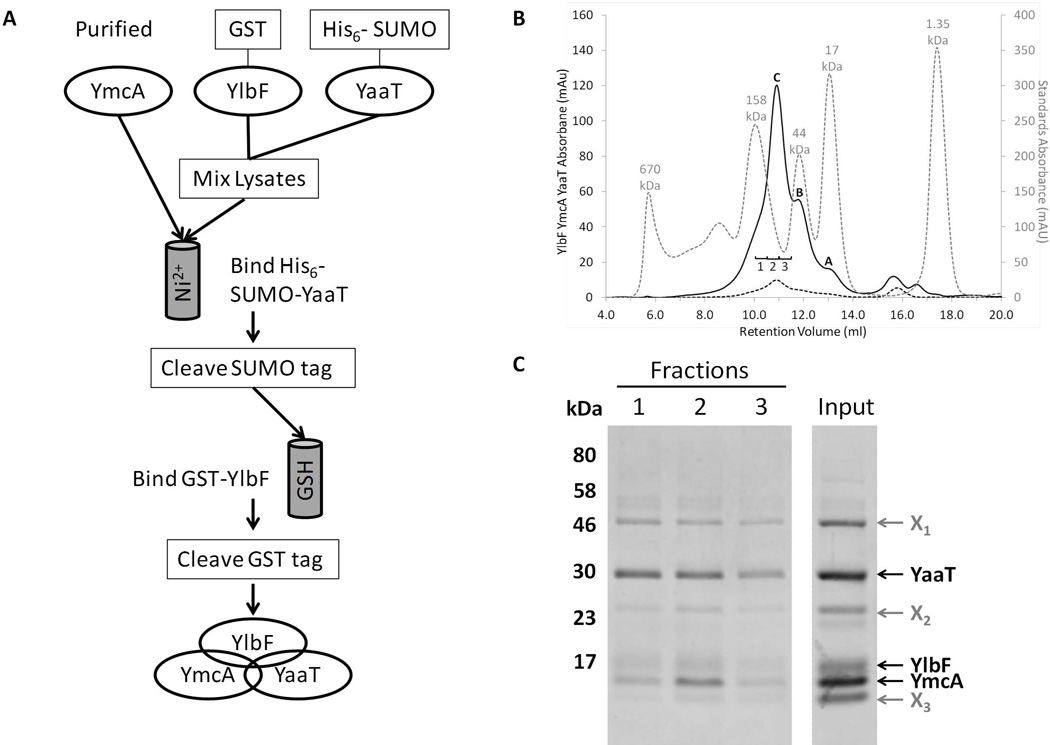
Figure 2.
YmcA, YlbF and YaaT form a ternary complex. (A) Co-purification scheme of the proteins from E. coli. First, GST-YmcA was expressed, purified, and the GST tag cleaved. Separate cultures of cells expressing GST-YlbF and His6-SUMO-YaaT were mixed and lysed together, and the lysates were incubated with the pure YmcA to allow for complex formation. This mixture was passed over nickel resin to isolate His6-SUMO-YaaT and associated proteins, and after cleavage of the SUMO tag the preparation was passed over glutathione (GSH) resin to isolate the protein complexes containing GST-YlbF. After cleavage of the GST-tag from YlbF, all three untagged proteins were present in the eluate. (B) Co-purified proteins were run on a Superose 12 size exclusion column, as described in Experimental procedures. Depicted are the sample absorbance at 215 nm (A215, solid black line) and absorbance at 280 nm (A280, dashed black line), which has a low intensity due to the presence of a single tryptophan residue in the complex (YaaT). Included for comparison is a mixture of molecular weight sizing standards (A280, dotted grey line). From left to right the standard peaks are: thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa), and vitamin B12 (1.35 kDa). Peaks A and B correspond to contaminating species that are roughly 20 and 45 kDa in size, respectively. Peak C represented the intact YmcA-YlbF-YaaT complex, and corresponds to ~80 kDa in size. (C) Fractions taken from peak C (labeled 1, 2, and 3) were TCA precipitated, resolved by SDS-PAGE on a 12.5% Tris-tricine gel and bands visualized by staining with Coomassie blue. The input lane contained 5 µg of partially purified protein complex. Bands labeled X1-X3 represented major contaminating proteins in the preparation. All samples shown were run on the same gel with irrelevant lanes removed.