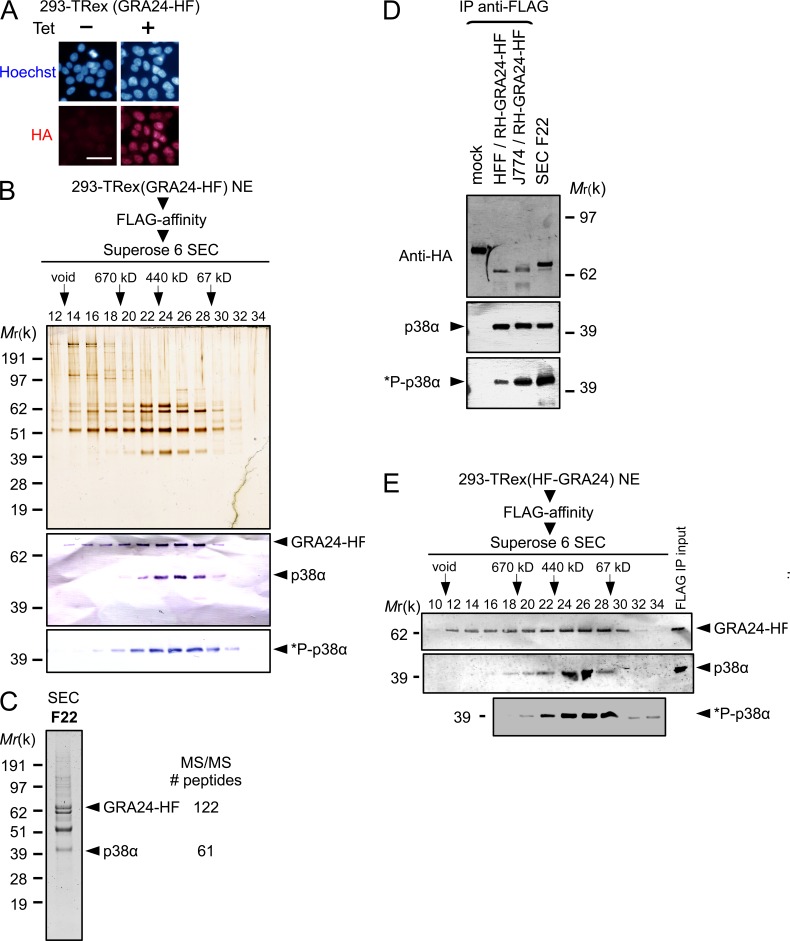
Figure 3.
GRA24 forms a dimeric complex with the p38α MAPK. (A) Immunofluorescence analysis (IFA) of GRA24-HAFlag ectopically and stably expressed in 293-TRex cell line. Cells were either left untreated (−) or treated with 1 µg/ml tetracycline for 20 h before fixation and staining with anti-HA antibodies (red) and Hoechst DNA-specific dye (blue). Bar, 2.5 µm. (B) GRA24-associated polypeptides were purified from nuclear extracts of 293-TRex cells tetracycline-induced to express GRA24-HF. Size exclusion chromatography (SEC) of GRA24-containing complexes after Flag affinity selection. Fractions were analyzed by silver staining and immunoblotting to detect GRA24-HF (anti-HA), total p38α, and Thr180/Tyr182 phosphorylated *P-p38α. Data are representative of two experiments. (C) Mass spectrometry analysis of SEC fraction 22. Identity of the proteins with their respective number of peptides is indicated on the right. (D) GRA24-p38α association detected in HFF and J774 MØ infected by RHku80 GRA24-HF (18 h). Tagged proteins were Flag-immunoprecipitated from extracts and eluates were analyzed by immunoblotting. Data are representative of two experiments. (E) GRA24-associated polypeptides were purified from nuclear extracts of 293-TRex cells induced to express an N-terminal HAFlag-tagged GRA24. SEC Fractions were analyzed by immunoblotting to detect HAFlag-GRA24 (anti-HA), total p38α, and Thr180/Tyr182 phosphorylated *P-p38α. Data are representative of two experiments.