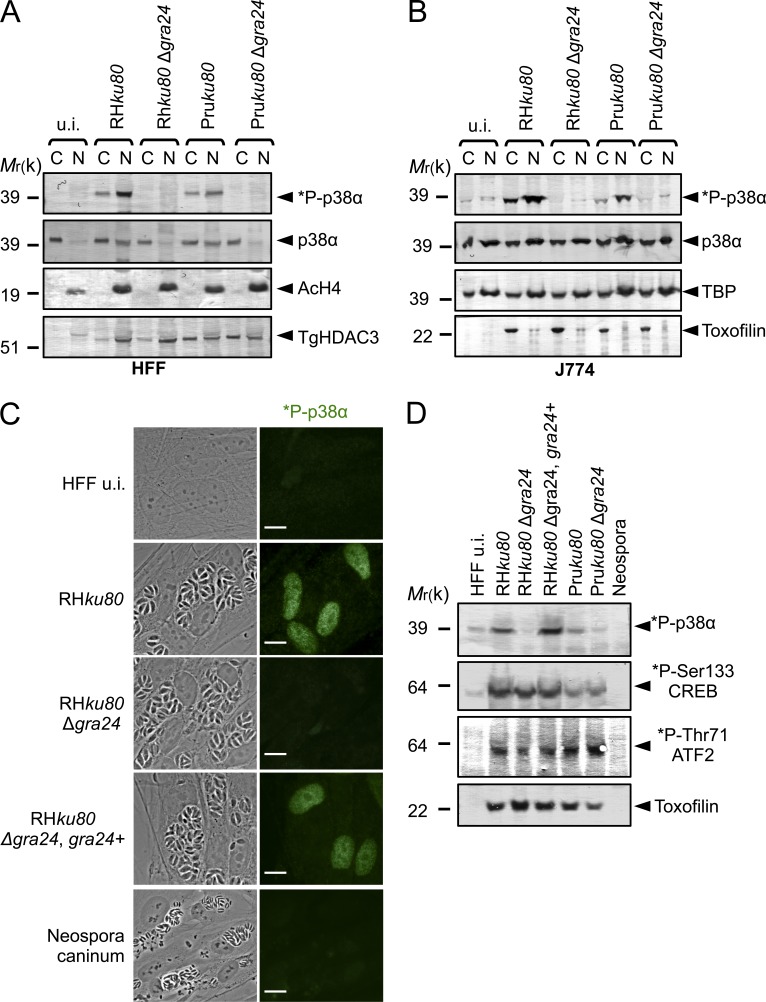
Figure 4.
GRA24 elicits phosphorylation and relocation of host p38α in the nucleus. (A and B) Immunoblotting detection of *P-p38α and p38α in HFF (A) and J774 MØ (B) uninfected (u.i.) or infected (24 h) with RHku80, RHku80 Δgra24, Pruku80, and Pruku80 Δgra24 strains. Cytosolic (C) and nuclear (N) cell lysates were probed with the indicated antibodies. Histone H4 acetylated (K5-K8-K12-K16) (nuclear), TBP (host-specific), and TgHDAC3 (parasite-specific) levels are shown as loading controls. (C) Subcellular in situ detection of *P-p38α (green) in HFF uninfected (u.i.) or infected (24 h) with RHku80, RHku80 Δgra24, RHku80 Δgra24, GRA24+, and Neospora caninum strains. Data are representative of at least three experiments. Bars, 10 µm. (D) Immunoblotting detection of *P-p38α, *P-Ser133-CREB, and *P-Thr71-ATF2 in nuclear fraction and Toxofilin (parasite-specific) in cytosolic fraction from HFF uninfected (u.i.) or infected (24 h) with the aforementioned strains and Neospora caninum. Data are representative of two experiments.