Figure 7.
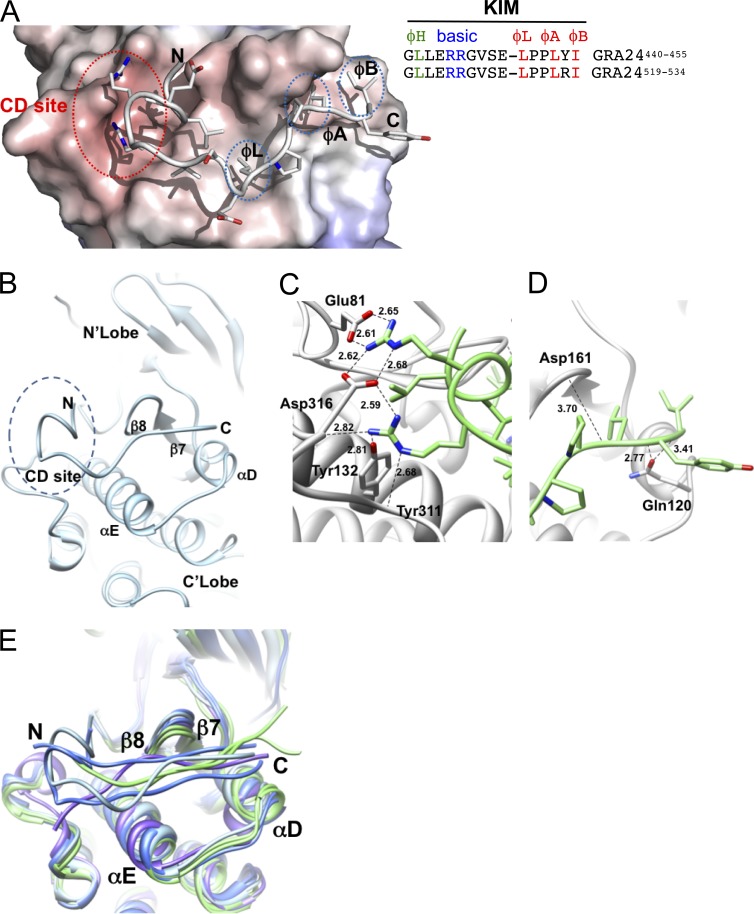
Structural features of the GRA24-KIM1 peptide main chain docked into p38α. (A and B) GRA24-KIM1 peptide docked into p38α (1LEW). The N-terminal side binds to the charged CD site while C-terminal hydrophobic residues insert into hydrophobic pockets ϕL, ϕA, and ϕB. The hydrophobic pocket is formed by the loop between β7 and β8 and the loop joining αD and αE helices. (C and D) GRA24-KIM1 peptide docked and energy minimized (see Materials and methods) into p38 KIM-binding domain. (C) Hydrogen bond network of the two arginines at the CD domain. (D) Peptide hydrophobic C terminus flanked by loops 159–163 and 118–125. (E) Cα-backbone KIM binding site area superposition of p38α, ERK2, and JNK models demonstrating local displacement upon binding. p38α peptides (MEF2A-1lew and MKK3b-1lez green), ERK2 peptides (MKP3-2fys and HePTP-2gph, blue), and JNK (pepJIP1-1ukh, purple) and p38α-GRA24/KIM1 docked peptide (light blue) are shown.