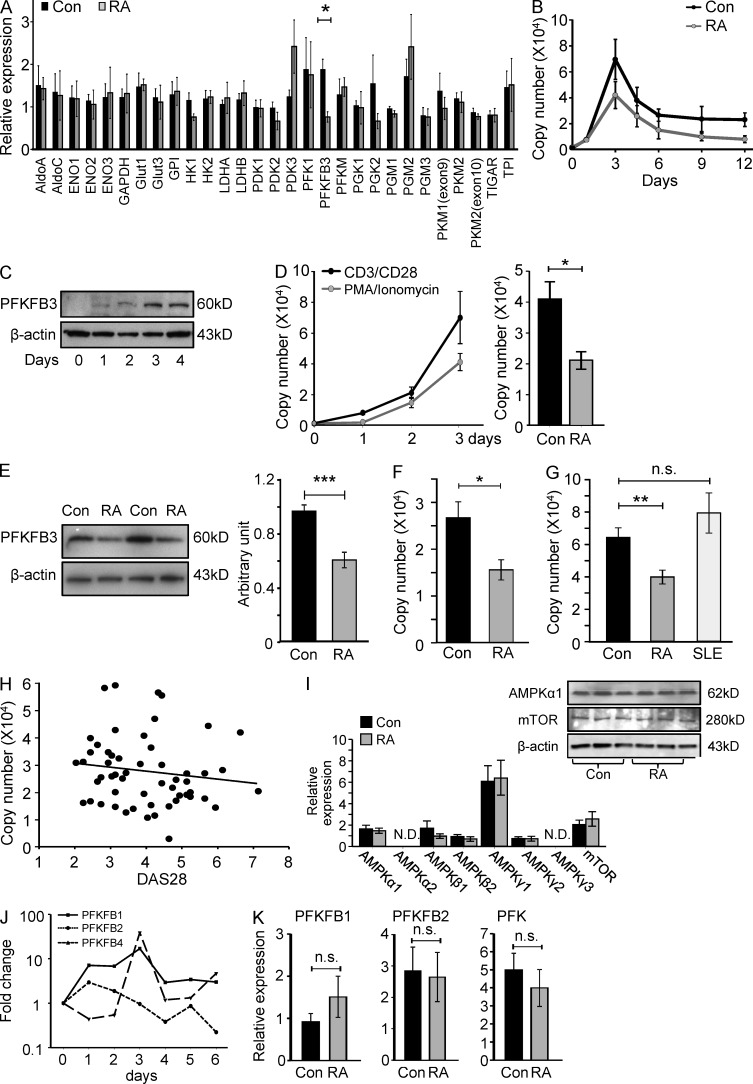
Figure 2.
PFKFB3 induction is suppressed in RA T cells. Naive CD4 (CD4+CD45RO−) T cells were isolated from RA patients and age-matched controls and stimulated with anti-CD3/CD28 microbeads for 3 d. Glycolysis-related gene transcripts were quantified by qPCR (A, n = 3). Kinetics of the expression of PFKFB3 in T cells following TCR ligation were monitored by RT-PCR over 12 d (B, 6 RA patients and 6 healthy controls) or by Western blotting over 4 d (C). Naive CD4 T cells were stimulated with either anti-CD3/CD28 microbeads or 20 ng/ml PMA and 200 µg/ml ionomycin and PFKFB3 transcript levels were monitored over 72 h in six samples. PMA/ionomycin-induced PFKFB3 transcripts from 5 RA patients and 6 controls are shown (D). Protein levels of PFKFB3 were quantified by Western blotting. Representative data for 2 patients and 2 controls are shown. Quantification of band densities from five independent experiments in 10 patients and 10 control samples are given as mean ± SEM (E). Expression of PFKFB3 in CD4+CD45RO+ memory T cells was quantified in 10 controls and 5 RA patients by RT-PCR (F). Activation-induced up-regulation of PFKFB3 was compared in control T cells, RA T cells, and SLE T cells on day 3 after TCR ligation. PFKFB3 mRNA levels were determined by RT-PCR in n = 16 RA patients, n = 33 controls, and n = 11 SLE patients. Results are given as mean ± SEM (G). PFKFB3 transcripts quantified by qPCR on day 3 after T cell stimulation were correlated with RA disease activity (DAS28; r2 = 0.020, P = 0.306; H). Expression of AMPK family members and mTOR was determined by qPCR and Western blotting, respectively. Data from 6 RA patients and 6 age-matched controls are presented as mean ± SEM and representative immunoblots are shown (I). Transcripts of PFKFB1, 2, and 4 were quantified by qPCR over 6 d (J). Activation-induced up-regulation of PFKFB1, 2, and 4 were compared in control and RA T cells (n = 8 each) on day 3 after TCR ligation. Results are given as mean ± SEM (K). *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., non-significant.