Figure 1. Electrophysiological Properties of UCP1 Current.
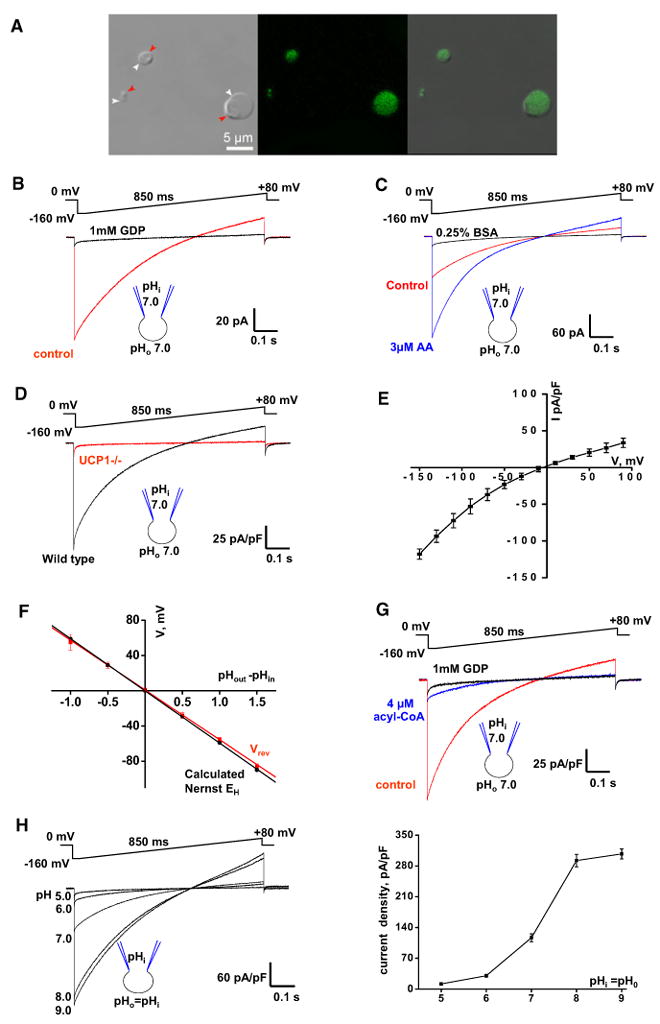
(A) Transmitted, fluorescent, and superimposed images (left to right) of BAT mitoplasts isolated from mice expressing CFP in the mitochondrial matrix (false green color). White arrows, IMM; red arrows, remnants of outer membrane.
(B) Whole-mitoplast putative UCP1 current before (control, red) and after (black) the addition of 1 mM GDP to the bath solution. The voltage protocol is indicated at the top. The pipette-mitoplast diagram indicates the recording conditions. The mitoplast membrane capacitance was 1.1 pF.
(C) Putative UCP1 current (control, red) is potentiated by 3 μM arachidonic acid (AA, blue) and inhibited by 0.25% BSA (black). The mitoplast membrane capacitance was 1.0 pF.
(D) Representative whole-mitoplast currents recorded from wild-type (black) and UCP1–/– (red) mitoplasts.
(E) Current-voltage dependence of IUCP1. Amplitudes were measured at the beginning of the voltage steps shown in Figure S1D; n = 5.
(F) IUCP1 reversal potentials (Vrev) compared to H+ equilibrium potentials (EH) predicted by the Nernst equation. The red line indicates the linear fitting of IUCP1 reversal potentials versus ΔpH; n = 3–10. The black line indicates EH calculated by the Nernst equation at 24°C.
(G) Whole-mitoplast IUCP1 before (control, red), after the addition of 4 μM oleoyl-CoA to the bath solution (blue), and after the subsequent application of 1 mM GDP (black).
(H) Left panel: IUCP1 at different symmetrical pH values. Representative traces recorded from different mitoplasts are shown. Right panel: Mean IUCP1 densities in different symmetrical pH values; n = 4–12. Amplitudes were measured upon stepping from 0 to [C0]160 mV as in the left panel.
Error bars represent standard error of the mean (SEM). See also Figure S1.
