Abstract
The development of T-cell responses specific for myeloma-associated antigens correlates with improved clinical outcomes in multiple myeloma patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation and donor lymphocyte infusions. Thus, immunotherapeutic strategies that further increase the frequency of Wilms tumor 1 (WT1)-specific T cells may provide clinical benefits to multiple myeloma patients.
Keywords: multiple myeloma, allogeneic stem cell transplantation, donor lymphocyte infusion, WT1-specific cytotoxic T cells, graft-vs.-myeloma effect
Allogeneic stem cell transplantation (alloSCT) remains a potentially curative option for multiple myeloma (MM) patients. Lower relapse rates have been reported for alloSCT as compared with autologous SCT, most likely due to the graft-vs.-myeloma (GvM) effect elicited by donor lymphocytes. However, both conventional and non-myeloablative allotransplants are associated with high rates of morbidity and mortality, most often resulting from graft- vs.-host disease (GvHD), post-transplantation infections or disease progression.1,2
The depletion of T cells from allografts prior to transplantation reduces GvHD and transplant-related mortality. However, this approach is believed to carry its own limitations, including a delayed immune reconstitution and a potentially greater risk of relapse due to the abrogation of immune GvM activity.3 On the other hand, allogeneic T cell-depleted hematopoietic SCT (alloTCD-HSCT), which does not require post-transplantation immunosuppressive therapy, provides a convenient platform for the subsequent implementation of immunotherapies, such as the infusion into patients of donor lymphocytes or antigen-specific T cells. In this setting, the lymphopenic environment of the host, as generated by pre-transplantation myelo/immunoablative conditioning, promotes the homeostatic proliferation of adoptively transferred T cells.4
An immune GvM effect has been documented in several clinical studies based on donor lymphocyte infusions (DLIs) post-alloSCT. Response rates of 40–60% have been reported in patients who received DLIs upon disease relapse after allogeneic transplantation. The ability of donor lymphocytes to mediate an effective antitumor response following allotransplantation is demonstrated by their capacity to frequently restore disease remission, and even induce continuous complete remission (CR) in some patients. However, the GvM responses observed in these studies were paralleled by a high incidence of acute and chronic GvHD. A strong association between GvHD and GvM effects has been observed, and GvHD has been suggested to constitute the major predictive factor for the of MM patients to DLI.5 This led to the initial assumption that the targets for GvHD and GvM activity are identical, in turn implying that a GvM effect cannot be achieved in the absence of a clinically significant GvHD.6 Recent results argue against this assumption and provide evidence for separate immune mechanisms being activated in the course of GvHD and GvM effects. Clinical remissions have indeed been documented in the absence of significant GvHD. Moreover, clearly distinguishable T-cell clones associated with GvHD and GvM effects have been identified by longitudinal TCR Vβ repertoire analyses post-DLI.7
One myeloma-associated antigen that may be the target of GvM effects is Wilms tumor 1 (WT1). The emergence of T cells specific for WT1 in patients subjected to alloSCT for the treatment of myeloid leukemia and hematologic malignancies has previously been correlated with decreases in circulating malignant cells and in the levels of WT1-coding mRNA as well as with a reduced risk of relapse and prolonged disease-free survival.8 In our studies, we sought to examine whether WT1-specific T cells are also able to mediate a GvM effect in patients affected high-risk and repeatedly relapsed MM undergoing alloTCD-HSCT followed by DLI.9 We hypothesized that the depletion of T cells from allografts would (1) reduce the risk of GvHD and treatment-related mortality and (2) allow for the transfer of reduced amounts of donor lymphocytes post-transplantation, perhaps leading to a selective GvM effect in the absence of GvHD.
WT1 expression levels were detected by immunohistochemistry in malignant CD138+ plasma cells of the bone marrow in all 15 MM patients monitored and correlated with prognosis. A low frequency of WT1-specific T cells was detected in MM patients prior to allotransplantation, and this frequency correlated with pre-transplantation disease load. The repeated administration of escalating doses of donor lymphocytes in the post-transplantation lymphopenic setting resulted in the expansion of WT1-specific T cells. The emergence of these T cells was associated with a reduction or stabilization of disease. In some cases, the expansion and persistence of functional WT1-specific T cells was associated with sustained clinical remissions. Importantly, such a GvM effect occurred in the absence of discernable GvHD.9 Therefore, strategies aimed at enhancing immune responses to WT1 may be of therapeutic benefits in patients with WT1-expressing myelomas and perhaps other WT1-expressing malignancies.
The absolute number of WT1-specific T cells that we detected in MM patients upon alloTCD-HSCT and DLI was significantly higher than that previously documented in leukemia patients receiving a vaccine targeting PR1 and WT1 (namely, 31 and 1 WT1-specific CD8+ T cells/μL peripheral blood, respectively).10 Such a marked gap in the magnitude of WT1-specific cytotoxic T lymphocyte (CTL) responses achieved in these settings may reflect intrinsic differences between leukemia and myeloma or may result from differences inherent to vaccination approaches as compared with the adoptive transfer of donor lymphocytes.
DLIs are not “immediate” therapeutic interventions. Time is indeed required for adoptively transferred T cells to encounter cognate antigens, become activated, proliferate in vivo and mediate antitumor effects. Such a delay between DLIs and the development of clinically meaningful T-cell responses should be taken into attentive consideration for selecting additional therapeutic regimens, in particular for choosing whether to administer chemotherapy or other therapeutic interventions that may exert immunosuppressive effects on adoptively transferred T cells. Sufficient time should indeed be allowed for DLIs to develop clinically significant T-cell responses, which may limit the applicability of this approach to rapidly progressive malignancies.
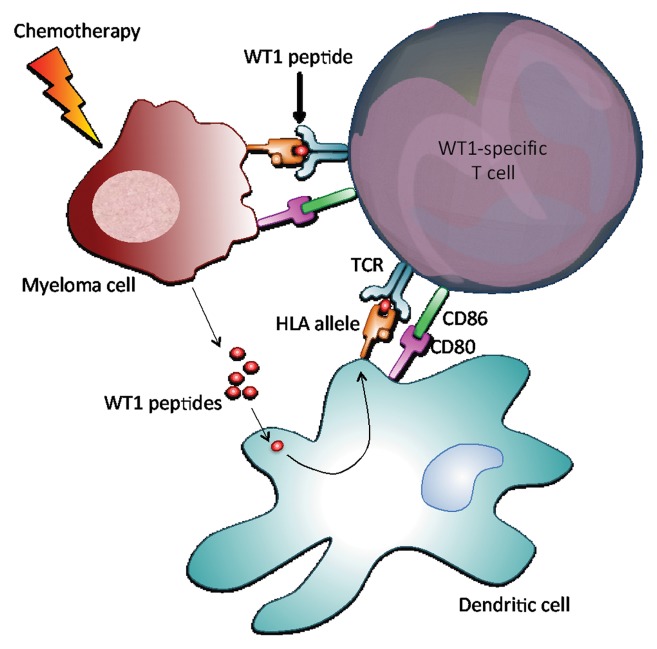
Conversely, the administration of immunomodulatory drugs such as lenalidomide prior to (or in combination with) DLIs facilitated the development of WT1-specific CTL responses in four of our patients who had not previously responded to DLIs alone. In this setting, the chemotherapy-induced death of myeloma cells is likely to promote the release of tumor-associated antigens and hence antigen uptake, processing and presentation by dendritic cells, overall favoring the development of tumor antigen-specific immune responses (Fig. 1).
Figure 1. Activation of WT1-specific T cells. Wilms tumor 1 (WT1)-expressing myeloma cells normally present immunogenic WT1 epitopes on their surface in the context of HLA molecules. In addition, the chemotherapy-induced death of myeloma cells results in the release of antigenic peptides, including WT1-derived peptides, which are taken up by dendritic cells, processed and subsequently presented to T cells, again in the context of HLA molecules. WT1-derived peptides complexed with HLA molecules are recognized by the T-cell receptor of WT1-specific T cells, resulting in T-cell activation and hence in the production of cytolytic mediators that exert graft-vs.-myeloma (GVM) effects.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24963
References
- 1.Gahrton G, Tura S, Ljungman P, Bladé J, Brandt L, Cavo M, et al. Prognostic factors in allogeneic bone marrow transplantation for multiple myeloma. J Clin Oncol. 1995;13:1312–22. doi: 10.1200/JCO.1995.13.6.1312. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, 3rd, et al. Blood Marrow Transplant Clinical Trials Network (BMT CTN) Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Heijst JW, Ceberio I, Lipuma LB, Samilo DW, Wasilewski GD, Gonzales AM, et al. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med. 2013;19:372–7. doi: 10.1038/nm.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3:668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 6.Huff CA, Fuchs EJ, Noga SJ, O’Donnell PV, Ambinder RF, Diehl L, et al. Long-term follow-up of T cell-depleted allogeneic bone marrow transplantation in refractory multiple myeloma: importance of allogeneic T cells. Biol Blood Marrow Transplant. 2003;9:312–9. doi: 10.1016/S1083-8791(03)00075-2. [DOI] [PubMed] [Google Scholar]
- 7.Orsini E, Alyea EP, Schlossman R, Canning C, Soiffer RJ, Chillemi A, et al. Changes in T cell receptor repertoire associated with graft-versus-tumor effect and graft-versus-host disease in patients with relapsed multiple myeloma after donor lymphocyte infusion. Bone Marrow Transplant. 2000;25:623–32. doi: 10.1038/sj.bmt.1702187. [DOI] [PubMed] [Google Scholar]
- 8.Rezvani K, Yong AS, Savani BN, Mielke S, Keyvanfar K, Gostick E, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood. 2007;110:1924–32. doi: 10.1182/blood-2007-03-076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyler EM, Jungbluth AA, O’Reilly RJ, Koehne G. WT1-specific T-cell responses in high-risk multiple myeloma patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation and donor lymphocyte infusions. Blood. 2013;121:308–17. doi: 10.1182/blood-2012-06-435040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–42. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]