Abstract
Advanced renal cancer is an incurable malignancy in need of novel therapeutic avenues. We have generated interferon γ (IFNγ)-based fusion antibodies (immunocytokines) that target CD70, a putative biomarker of renal cancer. These immunocytokines efficiently labeled renal cancer cells, and, when combined with the proteasome inhibitor bortezomib, killed them by activating a RIP1-dependent necrotic pathway.
Keywords: CD70, immunocytokines, interferon, necroptosis, necrosis, renal cell carcinoma, RIP1
Renal cell carcinomas (RCCs) cause over 100,000 deaths worldwide each year. Clear cell RCCs (ccRCCs) represent the dominant histological subtype of this cancer, accounting for over 4/5 of all RCC cases. Although early-stage RCC is well controlled by surgery, this cancer is largely asymptomatic, and about 1/3 of all patients present with locally advanced or metastatic disease at diagnosis. Unlike early-stage disease, metastatic RCC is chemotherapy-resistant, the most lethal of all genitourinary malignancies.1
Current frontline treatment options for metastatic RCC rely on small agents that target signaling nodes critical for cell growth, angiogenesis and nutrient sensing. Although most patients benefit from such pharmacological approaches, RCC cells rapidly become chemoresistant and virtually all patients relapse and succumb to metastatic disease within 2 to 5 y.2 Advanced RCC is therefore still in desperate need of novel therapeutic interventions.
Before the widespread use of targeted anticancer agents, cytokine-based immunotherapy— in particular, with interferon (IFN)α and interleukin (IL)-2—represented the primary treatment option for RCC. Up to 20% of patients with advanced RCC exhibited partial responses to immunotherapy, with durable responses and occasional cures reported in a smaller patient subset. Indeed, the curative ability of cytokine-based approaches represent a primary advantage of immunotherapy over chemotherapy, despite the severe side effects that often accompany the use of these biological agents in the clinic. To a large extent, the ability of cytokines to induce lasting remissions may stem from their capacity to activate multiple antitumor mechanisms. For example, IFNγ does not only exert immunomodulatory effects, but, similar to IFNα, also mediates antiangiogenic and tumoricidal functions.2
Promising results were obtained in early clinical trials testing IFNγ as a monotherapeutic strategy against RCC, but issues related to its systemic toxicity and disappointing results from a large Phase III trial dampened initial enthusiasm.2 We have previously found that IFNγ efficiently induced the necrotic death of proliferating cells devoid of NF-κB pro-survival signaling.3 As several nodes in the NF-κB signaling pathway are potentially druggable, the translational ramifications of these findings were clear, in particular as earlier clinical trials testing IFNγ failed to take into consideration the direct tumoricidal properties of this cytokine.2
We therefore sought to develop next-generation IFNγ-based immunocytokines that would deliver IFNγ to RCC cells for the express purpose of inducing their necrotic death. Immunocytokines—fusion proteins in which a fully functional cytokine is conjugated to an antibody—not only provide a mechanism for specifically targeting the tissue of interest, but also improve the clinical utility of cytokines by improving their stability in vivo.4 Typically, immunocytokines are created by fusing the desired cytokine to the C-terminus of the heavy chain of an intact antibody. This strategy not only results in an agent with two cytokine moieties per antibody, but also spatially locates the cytokine distal to antigen-binding sites, minimizing the possibility that the cytokine would hinder antibody-antigen interactions.4
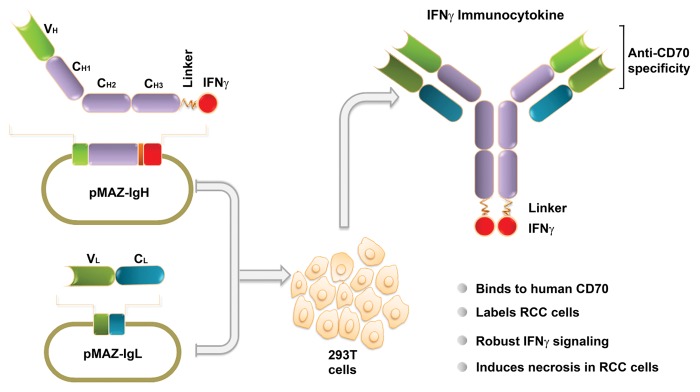
We have recently described the development and characterization of IFNγ-based immunocytokines targeting the putative RCC biomarker CD70 (Fig. 1).5 CD70 is expressed in virtually all metastatic ccRCC samples but is mostly undetectable in normal kidneys and expressed in a highly restricted manner in other normal tissues.6 By flow cytometry, we demonstrated that our immunocytokines specifically bound CD70 on the cell surface and robustly labeled a panel of human RCC cells. When evaluated for IFNγ activity, our immunocytokines exhibited species-specific signaling capability functionally indistinguishable from that of native, unconjugated IFNγ. Importantly, IFNγ-based immunocytokines mediated IFNγ-dependent cytotoxic effects against RCC cells in vitro, when combined with the proteasome inhibitor bortezomib.5 As one mechanism by which bortezomib mediates antitumor functions is via the inhibition of NF-κB,7 and as the bortezomib-mediated blockade of NF-κB signaling has previously been shown to sensitize RCC cells to multiple antineoplastic agents,2,8 our findings demonstrate that inhibiting NF-κB by bortezomib (or similar agents) can unmask the ability of IFNγ to directly kill RCC cells. In line with previous results,3 the cytotoxicity of IFNγ-based immunocytokines was limited by necrostatin-1, an inhibitor of receptor-interacting protein kinase 1 (RIP1), implicating a dominant role for RIP1-driven necrosis in this setting. Altogether, these observations set the stage for in vivo preclinical studies aimed at the deployment of IFNγ as a direct tumoricidal agent against RCC (for example, in combination with bortezomib).
Figure 1. Generation of interferon γ-based immunocytokines targeting human CD70. Two plasmids, one encoding interferon γ (IFNγ) fused to the C-terminus of an anti-CD70 IgG heavy chain (pMAZ-IgH), and one coding for the light chain of an anti-CD70 IgG (pMAZ-IgL), were co-transfected into human embryonic kidney HEK-293T cells, followed by the purification of immunocytokines from culture supernatants by protein A affinity chromatography. Immunocytokines produced in this manner efficiently bound CD70 and exerted robust IFNγ- dependent cytotoxic functions, demonstrating their potential as next-generation immunotherapeutics against renal cell carcinoma.
The strategy of targeting cytokines to tumor cells in order to maximally exploit their tumoricidal potential is not limited to IFNγ. Neutralizing NF-κB also sensitizes various malignant cells to apoptotic cell death triggered by IFNα,9 and IFNα-rituximab immunocytokines have already been shown to induce the apoptotic demise of tumor cells in vivo.10 Extending these findings, our unpublished results show that IFNα (and IFNβ) also induce necrosis when default survival signals are inhibited. Thus, both type I (α/β) and type II (γ) IFNs can engage multiple cytotoxic mechanisms if directly delivered to tumors. This suggests that combining IFN-based immunocytokines with agents that selectively neutralize pro-survival signaling pathways in tumor cells may provide clinical benefits to patients affected by any of the >20 highly-aggressive cancers for which IFNs are currently—or were previously—employed as therapeutic options.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24964
References
- 1.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran S, Adams GP. Interferon-γ-Induced Necrosis: An Antitumor Biotherapeutic Perspective. J Interferon Cytokine Res. 2013;33:171–80. doi: 10.1089/jir.2012.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thapa RJ, Basagoudanavar SH, Nogusa S, Irrinki K, Mallilankaraman K, Slifker MJ, et al. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol. 2011;31:2934–46. doi: 10.1128/MCB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17:583–90. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Chen P, Nogusa S, Thapa RJ, Shaller C, Simmons H, Peri S, et al. Anti-CD70 Immunocytokines for Exploitation of Interferon-γ-Induced RIP1-Dependent Necrosis in Renal Cell Carcinoma. PLoS One. 2013;8:e61446. doi: 10.1371/journal.pone.0061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boursalian TE, McEarchern JA, Law CL, Grewal IS. Targeting CD70 for human therapeutic use. Adv Exp Med Biol. 2009;647:108–19. doi: 10.1007/978-0-387-89520-8_7. [DOI] [PubMed] [Google Scholar]
- 7.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Morais C, Gobe G, Johnson DW, Healy H. The emerging role of nuclear factor kappa B in renal cell carcinoma. Int J Biochem Cell Biol. 2011;43:1537–49. doi: 10.1016/j.biocel.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Yang CH, Murti A, Pfeffer SR, Basu L, Kim JG, Pfeffer LM. IFNalpha/beta promotes cell survival by activating NF-kappa B. Proc Natl Acad Sci U S A. 2000;97:13631–6. doi: 10.1073/pnas.250477397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115:2864–71. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]