Abstract
Cardiac thrombus provides a substrate for embolic events and an indication for anticoagulant therapy. Cardiac magnetic resonance (CMR) imaging enables thrombus to be detected based on intrinsic tissue characteristics related to avascular tissue composition. Delayed enhancement CMR tissue characterization has been well validated for thrombus assessment using references of both pathology and clinical thromboembolic outcomes. Comparative studies have demonstrated CMR to yield improved thrombus detection compared to echocardiography, which typically detects thrombus based on anatomic appearance. Experimental studies have demonstrated the feasibility of targeted CMR contrast agents for assessing thrombus composition and chronicity. This review examines established and emerging literature on use of CMR for assessing cardiac thrombus.
Keywords: magnetic resonance imaging, thrombus, echocardiography


Introduction
Accurate detection of cardiac thrombus affects clinical outcomes and therapeutic management as thrombus provides a substrate for thromboembolic events and a rationale for anticoagulation. Cardiac magnetic resonance (CMR) imaging enables thrombus to be detected based on intrinsic tissue characteristics related to avascular tissue composition. CMR tissue characterization for thrombus has been well validated when compared to reference standards of both pathology and clinical outcomes. Recent comparative studies have demonstrated that CMR yields superior detection of left ventricular (LV) thrombus compared to echocardiography (echo), which detects thrombus based on anatomical appearance (i.e., morphology) rather than tissue characteristics. This review offers an overview of established and emerging literature concerning CMR tissue characterization for assessment of cardiac thrombus.
Imaging Approach
Delayed enhancement CMR (DE-CMR) is a technique widely used to differentiate between infarcted and viable myocardium based on relative differences in gadolinium-based contrast uptake and can be used to identify thrombus. Whereas gadolinium-based contrast agents demonstrate uptake within infarcted and, to a far lesser extent, viable myocardium, thrombus manifests an absence of gadolinium uptake due to its avascular composition.1 On DE-CMR, thrombus appears as a low signal intensity mass (attributable to the absence of contrast uptake) surrounded by high signal intensity (i.e., contrast-enhanced) structures such as cavity blood and/or surrounding myocardium. The absence of contrast enhancement can be used to distinguish thrombus from other masses such as neoplasm, which typically demonstrate contrast uptake due to tumor-associated vascularity.
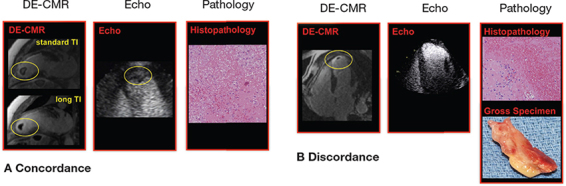
On conventional DE-CMR—which is tailored to null viable myocardium (typical inversion time 200-300 msec)—thrombus appears grey or “etched,” viable myocardium black, and infarcted myocardium white. Both viable myocardium and thrombus can appear relatively dark and may be difficult to distinguish from one another. DE-CMR can be further tailored for thrombus assessment by prolonging the inversion time (i.e., 600 msec) to selectively null avascular tissue such as thrombus. This so-called “long inversion time” approach produces an image that renders thrombus black and surrounding myocardium bright.1 Figure 1A provides a representative example of LV thrombus assessment by both standard and long inversion time DE-CMR.
Figure 1.
Representative examples of LV thrombus assessment by DE-CMR. Two representative examples of thrombus by DE-CMR tissue characterization as compared to (contrast-enhanced) transthoracic echo. (A) Large intracavitary thrombus within LV apex (yellow circle) concordantly identified by both DE-CMR and echo. Note that thrombus appears “etched” on standard DE-CMR (top, inversion time 250-350 msec) and homogenously black in long inversion time (bottom, inversion time = 600 msec) imaging. (B) Small mural thrombus (yellow circle) on standard DE-CMR, despite negative echo. For both examples, surgical resection enabled thrombus verification based on histopathology (right, hematoxylin and eosin stain, low power). Modified from JACC Cardiovasc Imaging, 2009. ©2009; reprinted with permission from Elsevier.8 LV: left ventricular; DE-CMR: delayed-enhancement cardiac magnetic resonance; echo: echocardiography.
CMR also can be used to assess structural risk factors for thrombus. DE-CMR is well validated for infarct quantification, yielding findings that closely agree with size and morphology of myocyte necrosis on histopathology.2, 3 Cine-CMR, typically acquired immediately prior to DE-CMR, provides a highly reproducible means of quantifying cardiac chamber geometry (i.e., size, aneurysmal deformation) and contractile function.4 Thus, within a single test, CMR enables both direct identification of thrombus (based on tissue characteristics) and quantification of structural risk factors that may predispose to thrombus formation.
Left Ventricular Thrombus
Validation
DE-CMR has been well validated for LV thrombus in several different at-risk cohorts. Among 160 patients undergoing LV reconstruction surgery (in whom pathology verification was uniformly available), Srichai et al. reported that CMR yielded more than a 3-fold higher diagnostic accuracy than did transthoracic echo (87% vs. 27%).5 Improved accuracy was predominantly attributable to markedly higher sensitivity for CMR as compared to echo (88% vs. 23%).
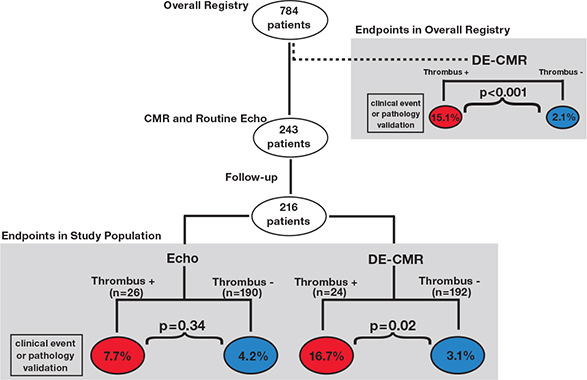
Both pathology and clinical endpoints have been used to validate LV thrombus assessment by DE-CMR in patients with heart failure. Among a registry of 784 patients with LV systolic dysfunction, patients with LV thrombus identified by DE-CMR had more than a 7-fold higher rate of validative endpoints (cerebrovascular accident, transient ischemic attack, or pathology verification of thrombus) during 6-month follow-up than did those without thrombus (15.1% vs. 2.1%, P <.001).6 Among a subgroup of patients in whom echo was performed clinically, DE-CMR tissue characterization again yielded improved endpoint stratification.7 Patients with LV thrombus identified by DE-CMR had more than a 5-fold higher rate of endpoints compared to those without thrombus (16.7% vs. 3.1%, P = .02), whereas echo yielded only a 1.8-fold difference (7.7% vs. 4.2%, P = .34) (Figure 2). Increased event rates occurred among patients with LV thrombus detected by DE-CMR despite the fact that these patients were more likely to be anticoagulated than those with thrombus detected by echo (63% vs. 36%, P = .054).
Figure 2.
Validative endpoints in relation to LV thrombus detection by DE-CMR. Outcomes-based assessment (cerebrovascular accident, transient ischemic attack, or pathology-verified thrombus) in relation to the diagnosis of thrombus as evaluated in an overall registry of heart failure patients (upper right) as well as a subgroup of patients undergoing both DE-CMR and echo (center). Stratification based on presence (red) or absence (blue) of LV thrombus by DE-CMR yielded over a 7-fold difference in endpoints among patients in the overall registry (P<.001) and a 5-fold difference in the echo sub-group (P = .02), whereas stratification by echo yielded a nonsignificant difference between groups (P = .34). Reprinted from JACC Cardiovasc Imaging, 2011. ©2011; reprinted with permission from Elsevier.7 LV: left ventricular; DE-CMR: delayed-enhancement cardiac magnetic resonance; echo: echocardiography.
Taken together, these data incorporating both pathology as well as clinical embolic events support the use of DE-CMR tissue characterization as a noninvasive reference for LV thrombus.
Performance Characteristics of Echocardiography
DE-CMR has been used as a noninvasive reference to test diagnostic performance of echo for LV thrombus. Among a mixed cohort of heart failure and post-myocardial infarction patients, multiple imaging approaches that detect thrombus based on anatomic appearance were compared to a reference of DE-CMR tissue characterization (Table 1).8 In this multimodality research protocol, nearly two-thirds of thrombi detected by DE-CMR were missed by noncontrast echo (sensitivity 33%). While both contrast echo and cine-CMR yielded improved performance versus noncontrast echo, both modalities missed nearly one-third of thrombi detected by DE-CMR (sensitivity 61-79%). Thrombi detected by DE-CMR but missed by echo were more likely to be mural in shape (P <.05) or, when protuberant, small in size (P = .02). Figure 1 provides representative examples of comparative thrombus assessment by DE-CMR and echo, with the two modalities demonstrating concordance for large intracavitary thrombus (1A) and DE-CMR demonstrating incremental utility for detection of small mural thrombus (1B).
Table 1.
Diagnostic performance of routine imaging for LV thrombus. Includes echo and cine-CMR calculated using DE-CMR as the standard for LV thrombus.
| Sensitivity | Specificity | Accuracy | Positive Predictive Value | Negative Predictive Value | |
| Noncontrast Echo | 33% | 94% | 82% | 57% | 85% |
| Contrast Echo | 61% | 99% | 92% | 93% | 91% |
| Cine-CMR | 79% | 99% | 95% | 95% | 95% |
Modified from JACC Cardiovasc Imaging, Vol. 2, Weinsaft et al. ©2009; reprinted with permission from Elsevier.8 LV: left ventricular; Echo: echocardiography; CMR: cardiac magnetic resonance; DE: delayed enhancement.
DE-CMR also has been used to study echo performance in routine clinical practice, in which echo is typically used as a screening test for cardiac function/anatomy rather than for the primary purpose of LV thrombus evaluation. Among 243 patients with systolic dysfunction, performance of routine clinical echo for LV thrombus varied markedly based on clinical indication for imaging: Sensitivity increased more than two-fold (60% vs. 26%) and positive predictive value more than three-fold (75% vs. 21%) for echoes performed to assess LV thrombus compared with those performed for non-thrombus indications.7 Image quality impacted echo performance, as evidenced by higher reader-assigned diagnostic confidence scores (P <.02) for echoes read concordantly with DE-CMR regarding the presence or absence of LV thrombus.
Structural Risk Factors
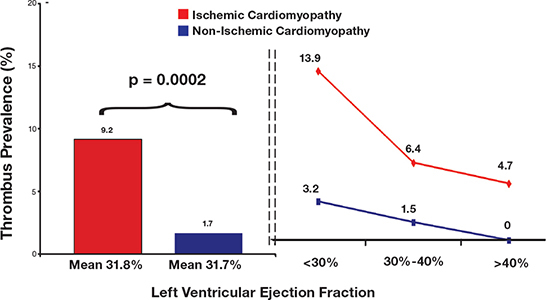
CMR has proven useful in identifying structural risk factors that predispose to LV thrombus. Myocardial scar burden (i.e., infarct size), another parameter quantified by DE-CMR, has been shown to be independently associated with LV thrombus. Among patients with systolic dysfunction, LV thrombus prevalence detected by DE-CMR was five-fold higher in patients with ischemic versus nonischemic cardiomyopathy (9.2% vs. 1.7%, P = .002) despite near identical LV ejection fraction (31.8 ± 10.5% vs. 31.7 ± 11.6%, P = .88) between groups (Figure 3).6 Myocardial infarct size paralleled thrombus prevalence and was 3-fold higher among ischemic versus non-ischemic patients (19.4% vs. 6.4% LV myocardium, P <.001). In multivariate analysis, thrombus was independently associated with myocardial infarct size (OR = 1.02 [CI 1.002 – 1.04] per % LV transmural infarction, P = .03) even after controlling for conventional risk factors including LV ejection fraction (OR = 0.94 [CI 0.92 – 0.97], P <.001).
Figure 3.
LV thrombus prevalence according to etiology and severity of myopathic dysfunction. LV thrombus prevalence (bar graph, left) was more than 5-fold higher among patients with ischemic cardiomyopathy (red) compared to those with nonischemic (blue) cardiomyopathy (9.2% vs. 1.7%) despite nearly identical LV ejection fraction. When each group was stratified according to ejection fraction (line graph, right), prevalence was higher in patients with ischemic disease for every ejection fraction group. Reprinted from J Am Coll Cardiol, 2008. ©2008; reprinted with permission from Elsevier.6 LV = left ventricular.
Myocardial infarct size and distribution have each been linked to LV thrombus following acute myocardial infarction (MI). Among a cohort of 200 patients with acute MI undergoing baseline (within 1 week) and follow-up (at 4 months) CMR, Delewi et al. reported that all LV thrombi occurred in patients with anterior infarctions.9 In multivariate analysis, LV thrombus on baseline CMR was independently associated with infarct size (B = .02, SE = .02, P <.001) and anterior infarction (B = 19.47, SE = 4900, P <.001). At follow-up, LV thrombus was again independently associated with infarct size (B = .06, SE = .02, P <.001). These findings parallel earlier results by Mollet et al., who studied LV thrombus in patients with ischemic heart disease and demonstrated an association between LV thrombus and hyperenhancement (i.e., infarction) within the vascular distribution of the left anterior descending artery.10 Regions with myocardial infarction were associated with a higher number of adjacent thrombi than were those without infarction (P = .002)
Together these multiple studies incorporating both heart failure and acute MI cohorts demonstrate a consistent association between infarct size and LV thrombus. Future research may address whether DE-CMR can be used to improve anticoagulant treatment strategies for patients with known or predisposing risk factors for LV thrombus.
Other Cardiac Thrombi
Whereas DE-CMR has been well validated for LV thrombus, large-scale studies are lacking concerning its utility for assessing thrombus in other chambers of the heart. Pilot studies have demonstrated that the left atrial appendage, a region particularly predisposed to thrombus formation, can be well visualized by DE-CMR.11 DE-CMR tissue characterization has been shown to differentiate between left atrial thrombus and neoplasm, as demonstrated in a mixed cohort including two patients with left atrial appendage thrombus.12 Similar to LV thrombus, both left atrial appendage size and velocity—potential structural risk factors for thrombus—can be assessed by CMR.13, 14 However, head-to-head comparisons between DE-CMR and the established clinical reference of transesophageal echo for actual identification of thrombus are lacking.
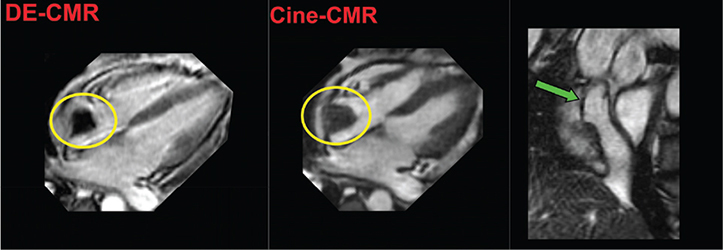
Right-sided thrombi, which can occur in the context of advanced heart failure and/or instrumentation, can also be visualized by DE-CMR (Figure 4).15, 16 Thrombus mobility as well as atrial arrhythmias (which also can be associated with left atrial appendage thrombus) can compromise CMR-based ECG gating, adding to the clinical challenges of DE-CMR for this purpose.
Figure 4.
Right atrial thrombus. Representative example of right atrial thrombus (yellow circle) identified by CMR. Both DE-CMR (left) and cine-CMR (right) demonstrate an irregularly contoured mass within the right atrium. Note that cine-CMR also demonstrates the mass to be contiguous with a central venous catheter (green arrow). On DE-CMR (long inversion time), the mass demonstrates absence of contrast uptake with associated homogenous black appearance, consistent with tissue characteristics of LV thrombus (see Figure 1). CMR: cardiac magnetic resonance; DE: delayed-enhancement; LV: left ventricular.
Emerging CMR Approaches – Novel Contrast Agents
One developmental focus of CMR is the use of novel targeted contrast for further characterization of thrombus. Fibrin-binding protein has been coupled with gadolinium chelates and tested for thrombus assessment. Vymazal et al., studying 38 patients, demonstrated that a fibrin-specific contrast agent can identify both cardiac and systemic vascular thrombus on CMR.17 Other contrast agents targeting fibrin-mediated processes have been tested as a means of distinguishing between acute and chronic thrombus. In an experimental study including both laboratory animals and human tissue histopathology specimens, Miserus et al. tested whether an α2-antiplasmin (α2AP) based gadolinium chelate could be used as a marker of thrombus chronicity.18 Among animals with experimentally induced carotid thrombi, α2AP-bound gadolinium demonstrated enhancement on CMR performed immediately after thrombus formation and no enhancement on CMR performed 24-48 hours thereafter. Among human pulmonary thromboemboli samples, α2AP levels were highest in fresh thrombi and demonstrated a time-dependent decrement, whereas fibrin levels remained elevated independent of thrombus age.
Like fibrin, platelets play an integral role in thrombus formation and offer a target for thrombus-specific contrast agents. Antibodies capable of binding the activated glycoprotein αIIbβ3 platelet binding site (gpIIb/IIIa) have demonstrated thrombus uptake.19 Using a murine model of carotid thrombosis, Klink et al. demonstrated that a gpIIb/IIIa-based agent (P975) bound to gadolinium chelates manifested persistent enhancement 2 hours following administration, suggesting its utility for delayed thrombus imaging as compared to conventional gadolinium (which typically manifests myocardial/cardiac washout within 30 minutes). Non-gadolinium-based contrast agents also have been used to target platelets. In a study of in-vivo murine and ex-vivo human carotid specimens, von zur Muhlen et al. reported that a contrast agent consisting of micro particles of iron oxide in conjunction with gpIIb/IIIa antibodies yielded improved mural thrombus detection as compared to control (P <.01).20
To date, clinical performance of targeted contrast agents for thrombus have not yet been reported in large-scale cohorts.
Conclusions
As the prevalence of heart failure and coronary artery disease continues to increase, the clinical importance of accurate diagnostic imaging for thrombus is heightened. Although echo is widely available, it can be diagnostically limited given its reliance on the anatomic appearance of thrombus, even when image quality is judged to be optimal. DE-CMR provides tissue characterization of thrombus and has been shown to improve LV thrombus detection compared to echo-based anatomic imaging. CMR also identifies structural risk factors for LV thrombus, including infarct size/distribution and contractile dysfunction. Novel CMR techniques, including use of targeted contrast agents, may further refine thrombus characterization. Future studies are anticipated to broaden the utility of CMR in the evaluation of cardiac thrombi.
Funding Statement
Funding/Support: Dr. Weinsaft has a sponsored research agreement with Lantheus Medical Imaging.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Contributor Information
Parag Goyal, Weill Cornell Medical College, New York, New York
Jonathan W. Weinsaft, Weill Cornell Medical College, New York, New York
References
- 1.Shah DJ, Judd RM, Kim J. Myocardial viability. In: Edelman RR Hesselink JR, Zlatkin M, Crues JV, editors. Clinical magnetic resonance imaging. 3rd edition. New York, NY: Elsevier; 2006. p. 1030-49. [Google Scholar]
- 2.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999 Nov 9;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 3.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000 Nov 15;36(6):1985–91. doi: 10.1016/s0735-1097(00)00958-x. [DOI] [PubMed] [Google Scholar]
- 4.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002 Jul 1;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 5.Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006 Jul;152(1):75–84. doi: 10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008 Jul 8;52(2):148–57. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 7.Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, et al. LV thrombus detection by routine echocardiography: Insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging. 2011 Jul;4(7):702–12. doi: 10.1016/j.jcmg.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging. 2009 Aug;2(8):969–79. doi: 10.1016/j.jcmg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delewi R, Nijveldt R, Hirsch A, Marcu CB, Robbers L, Hassell ME, et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol. 2012 Dec;81(12):3900–4. doi: 10.1016/j.ejrad.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002 Dec 3;106(23):2873–6. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen T, Chinitz JS, Spincemaille P, Min JK, Lerman BB, Prince MR, et al. Improved mri assessment of the left atrial appendage using delayed enhancement imaging as compared to black blood fast spin echo imaging. 16th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine. Toronto: Ontario; 2008. [Google Scholar]
- 12.Hong YJ, Hur J, Kim YJ, Lee HJ, Nam JE, Kim HY, et al. The usefulness of delayed contrast-enhanced cardiovascular magnetic resonance imaging in differentiating cardiac tumors from thrombi in stroke patients. Int J Cardiovasc Imaging. 2011 Dec;27 Suppl 1:89–95. doi: 10.1007/s10554-011-9961-8. [DOI] [PubMed] [Google Scholar]
- 13.Beinart R, Heist EK, Newell JB, Holmvang G, Ruskin JN, Mansour M. Left atrial appendage dimensions predict the risk of stroke/TIA in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011 Jan;22(1):10–5. doi: 10.1111/j.1540-8167.2010.01854.x. [DOI] [PubMed] [Google Scholar]
- 14.Muellerleile K, Sultan A, Groth M, Steven D, Hoffmann B, Adam G, et al. Velocity encoded cardiovascular magnetic resonance to assess left atrial appendage emptying. J Cardiovasc Magn Reson. 2012 Jun 21;14:39. doi: 10.1186/1532-429X-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza DD, Nguyen TD, Pua BB, Weinsaft JW. A young man with heart failure diffuse cardiac thrombi, and stroke. J Thorac Imaging. 2010 Nov;25(4):W128–30. doi: 10.1097/RTI.0b013e3181d042ff. [DOI] [PubMed] [Google Scholar]
- 16.Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ. 2012 Mar;21(3):185–8. doi: 10.1016/j.hlc.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, et al. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Invest Radiol. 2009 Nov;44(11):697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 18.Miserus RJ, Herías MV, Prinzen L, Lobbes MB, Van Suylen RJ, Dirksen A, et al. Molecular MRI of early thrombus formation using a bimodal alpha2-antiplasmin-based contrast agent. JACC Cardiovasc Imaging. 2009 Aug;2(8):987–96. doi: 10.1016/j.jcmg.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Klink A, Lancelot E, Ballet S, Vucic E, Fabre JE, Gonzalez W, et al. Magnetic resonance molecular imaging of thrombosis in an arachidonic acid mouse model using an activated platelet targeted probe. Arterioscler Thromb Vasc Biol. 2010 Mar;30(3):403–10. doi: 10.1161/ATVBAHA.109.198556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von zur Muhlen C, von Elverfeldt D, Moeller JA, Choudhury RP, Paul D, Hagemeyer CE, et al. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation. 2008 Jul 15;118(3):258–67. doi: 10.1161/CIRCULATIONAHA.107.753657. [DOI] [PubMed] [Google Scholar]


