Abstract
Background/Aims
Small core biopsy samples can occasionally be obtained with conventional endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA). Although most studies have focused on the cytological analysis of specimens, data regarding histological assessment is scarce. The aim of this study was to determine whether core biopsies by conventional EUS-FNA could increase the accuracy of EUS-guided sampling when combined with cytology in the absence of an on-site cytopathologist.
Methods
In the 95 consecutive patients (98 lesions) undergoing EUS-FNA of solid pancreatic masses and intra-abdominal lymphadenopathy, tissue coils from the needle were harvested for histology, and residual tissue was examined by cytology.
Results
Adequate samples were obtained by EUS-FNA cytology, histology, and combined cytology-histology in 91.8%, 65.3%, and 94.8% of patients, respectively. From the pancreas (n=67), adequate samples for histology were obtained by EUS-FNA in 68.7% of cases, compared with 58.0% from non-pancreatic cases (n=31), respectively (p>0.05). The overall sensitivity and accuracy of EUS-FNA was 78.0% and 81.6% for cytology alone, 63.4% and 69.4% for histology alone, and 84.1% and 86.7% for combined cytology-histology, respectively.
Conclusions
Combined cytology and histology analysis for diagnosing pancreatic masses and intra-abdominal lymphadenopathy may increase the diagnostic yield of conventional EUS-FNA without on-site cytology.
Keywords: Endoscopic ultrasound-guided fine needle aspiration, Histology, Cell biology, Pancreas
INTRODUCTION
In patients presenting with suspicious mass lesions of the pancreas, biliary tree, and intra-abdominal lymphadenopathy by cross-sectional imaging methods such as abdominal computed tomography (CT) and magnetic resonance imaging, tissue sampling is usually required to confirm or exclude the presence of malignancy. Fine needle aspiration (FNA) is extremely helpful in diagnosing these malignancies. Endoscopic ultrasonography (EUS)-guided FNA has become a mainstream technique for tissue acquisition from submucosal lesions arising from the gut, pancreatic masses, and lymph nodes directly adjacent to the gastrointestinal (GI) tract.
Support for the use of EUS over other tissue acquisition methods has been increasing because of its low morbidity, mortality, and cost.1-3 As rapid improvements occur in noninvasive diagnostic radiological modalities, it is vital for endosonographers to develop sampling techniques that can maximize tissue acquisition, sensitivity, and specificity while minimizing cost. However, cytological analysis of EUS-FNA specimens may have some disadvantages, such as a limited yield, especially when distinguishing between different tumor types is required. Furthermore, an on-site cytopathologist is needed to increase the diagnostic yield of EUS-FNA, which is not available in Korea. Endoscopic ultrasound-guided Trucut biopsy (EUS-TCB) has recently emerged as a method that seeks to overcome the limitations of EUS-FNA, in which a core tissue specimen is harvested to increase the yield. However, this method had some limitations such as low diagnostic yield, technical difficulty through the transduodenal route, and an increased risk of complications.4 Moreover, small core biopsies can be obtained with conventional EUS-FNA. Although most studies have focused on the cytology of specimens, few data exist regarding histological assessment. The aim of this study was to determine whether core biopsies obtained by conventional EUS-FNA could increase the accuracy of EUS-guided sampling when combined with cytology in the absence of an on-site cytopathologist.
MATERIALS AND METHODS
This study was a retrospective case review of all patients who underwent EUS-FNA from June 2008 through July 2010 by a single endoscopist at the Wonkwang University Hospital. All of the patients had pancreatic mass lesions and intra-abdominal lymphadenopathy lesions (20 mm or greater) that were accessible through the stomach and duodenum, with no involvement of the adjacent vascular structures. Informed consent was obtained from all patients before the procedure. The collection of data for this study was approved by our Institutional Review Board.
1. Performance of EUS-FNA
With the patient under sedation, EUS for guided puncture of the lesion was performed by using a GF-UCT 240 linear-array echoendoscope (Olympus Corp., Tokyo, Japan). We used 22- or 25-gauge needles (EUS N1; Cook Endoscopy, Winston-Salem, NC, USA). EUS-FNA was performed with a standardized approach by a single experienced investigator. The technique consisted of at least two FNA punctures of the lesion. If insufficient material had been obtained according to the macroscopic assessment of the investigator, passes were continued up to a maximum number of 5. Suction with a 5 mL prefixed suction syringe while moving the needle to and fro within the lesion was primarily applied in all cases, and this was changed to either forceful manual suction or no suction for the second pass in cases in which no material or too much aspirated blood was extruded. The stylet was introduced into the needle, and the extruded material was placed onto glass slides for primary inspection. The aspirated material was carefully smeared onto glass slides and fixed in an 96% alcohol solution for cytologic analysis. When an adequate tissue coil was successfully retrieved from the same needle, it was subsequently placed into a 4% formalin solution for histologic analysis (Fig. 1A). No on-site cytopathologist was available for slide review.
Fig. 1.
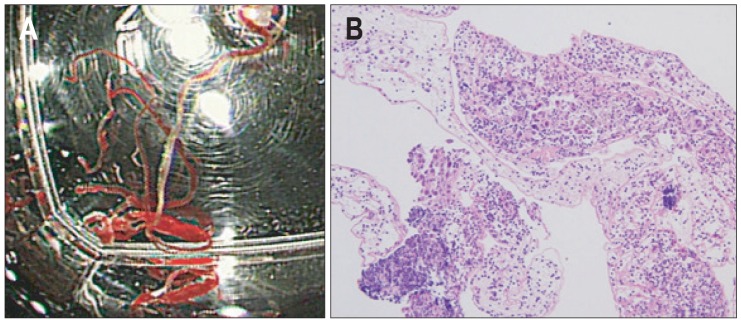
(A) Histologic core specimen in a formalin vial and (B) histological evaluation of a pancreatic sample obtained by endoscopic ultrasound-guided fine needle aspiration (H&E stain, ×400).
2. Histologic analysis
Formalin-fixed tissue specimens from all patients were embedded in paraffin for histologic analysis. Sections stained with hematoxylin and eosin and periodic acid-Schiff were examined to evaluate decisive histologic features (Fig. 1B). Immunohistochemical studies were performed only in cases of unclear histogenesis, for example, for tumors in which neuroendocrine differentiation was suspected. Both malignant lymphoma and endocrine tumors were categorized as malignancies according to our cytopatholgical diagnosis. One experienced GI histopathologist evaluated the specimens.
3. Classification of results
The diagnostic categories that were included in this study were the following: positive for malignancy, suspicious for malignancy, neoplasm, atypical, negative for malignancy, other (descriptive diagnosis), and unsatisfactory. The cytologic and histologic findings were classified as negative for malignancy if the diagnosis was negative for malignancy, atypical, or unsatisfactory. Positive cases were categorized as unequivocally positive for malignancy, neoplasm, or suspicious for malignancy. The acquired material was considered adequate for cytologic examination if it contained cells from the target organ and was adequate for histologic examination when it contained a coherent tissue specimen from the target organ. Specimens that contained inadequate material were not excluded from our analysis of diagnostic discrimination values, but were considered negative in the sense that they could not provide a diagnosis of malignancy (i.e., in an intention-to-diagnose analysis).
The diagnostic efficacy of smear cytology and histology of the needle core biopsies were compared. The final diagnosis was based on the integration of cytohistological findings, surgical pathology, and clinical course for more than 6 months.
4. Statistical analysis
Continuous variables were reported as mean±SD or median and range, if the data were not normally distributed. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the smear method and the histology of the needle core biopsies for the diagnosis of malignancy were calculated using the final cytology results or surgical pathology as the gold standards, including a 95% confidence interval (CI). The diagnostic performance of the two methods was compared by using the McNemar chi-square test with continuity correction. A p-value less than 0.05 was considered statistically significant.
RESULTS
A total of 95 patients were included in the study with 98 total lesions (Table 1). There were 61 males and 34 females, with a mean age of 65.0±10.8 years (range, 42 to 90 years). All lesions were technically accessible by EUS-FNA. The mean number of FNA passes was three (range, 1 to 6). Masses were located in the pancreas (n=67, 68.4%) and intra-abdominal lymphadenopathy occurred in 31 patients (31.6%). The mean tumor size was 2.7 cm (range, 1.5 to 5 cm); approximately 47.6% of lesions were below 3 cm, and 52.3% were 3 cm or larger. No significant complications that required any reintervention or specific measures were encountered.
Table 1.
Patient Characteristics and Technical Aspects of Tissue Sampling
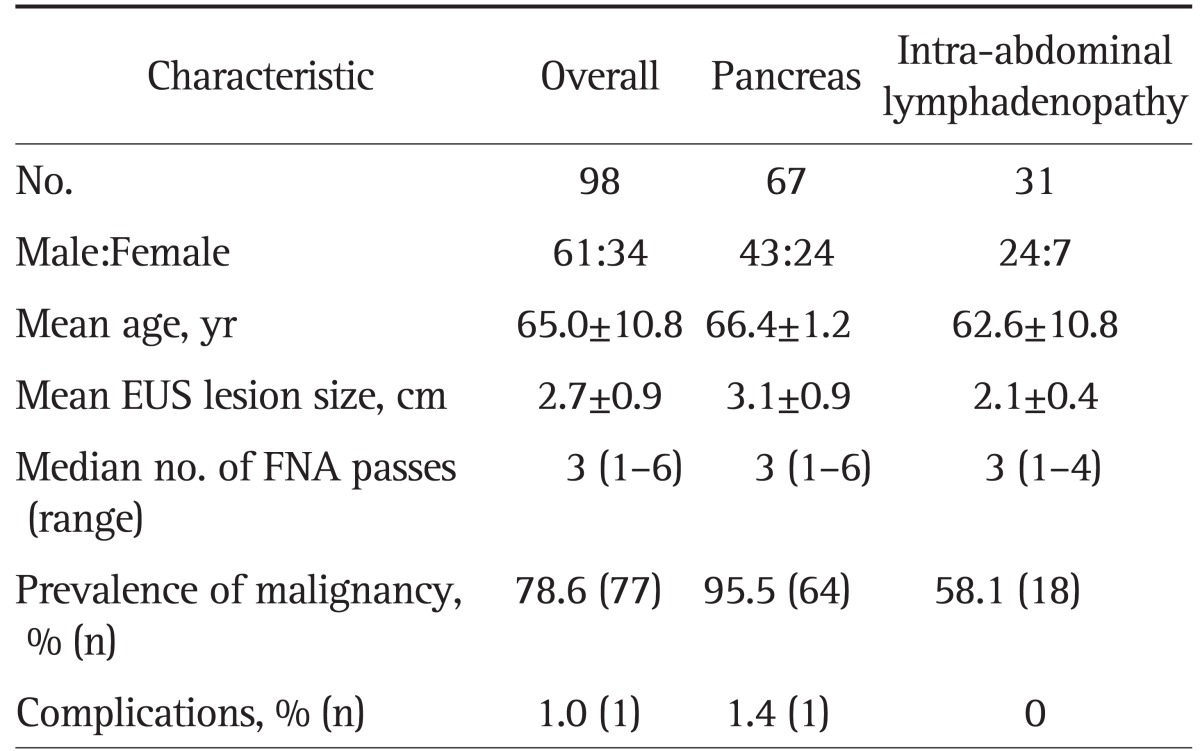
Data are presented as mean±SD.
EUS, endoscopic ultrasound; FNA, fine needle aspiration.
The final diagnoses, as well as the methods of diagnostic confirmation, are listed in Table 2; neoplasia (mostly adenocarcinoma) was found in 76 of 98 (77.6%) of the study cases. The underlying malignancy in the cases of intra-abdominal malignant lymphadenopathy was gallbladder cancer in 10 cases, extrahepatic cholangiocarcinoma in seven cases, and one case of lymphoma. Focal chronic pancreatitis was the final diagnosis in three cases, which was ascertained by operative histology (n=1) or a 12-month follow-up (n=2). Benign intra-abdominal lymphadenopathy was the final diagnosis in 13 cases, which was determined either by operative histology (n=5) or a follow-up of at least 6 months (n=8; range, 6 to 25 months).
Table 2.
Final Diagnosis of the Study Cases
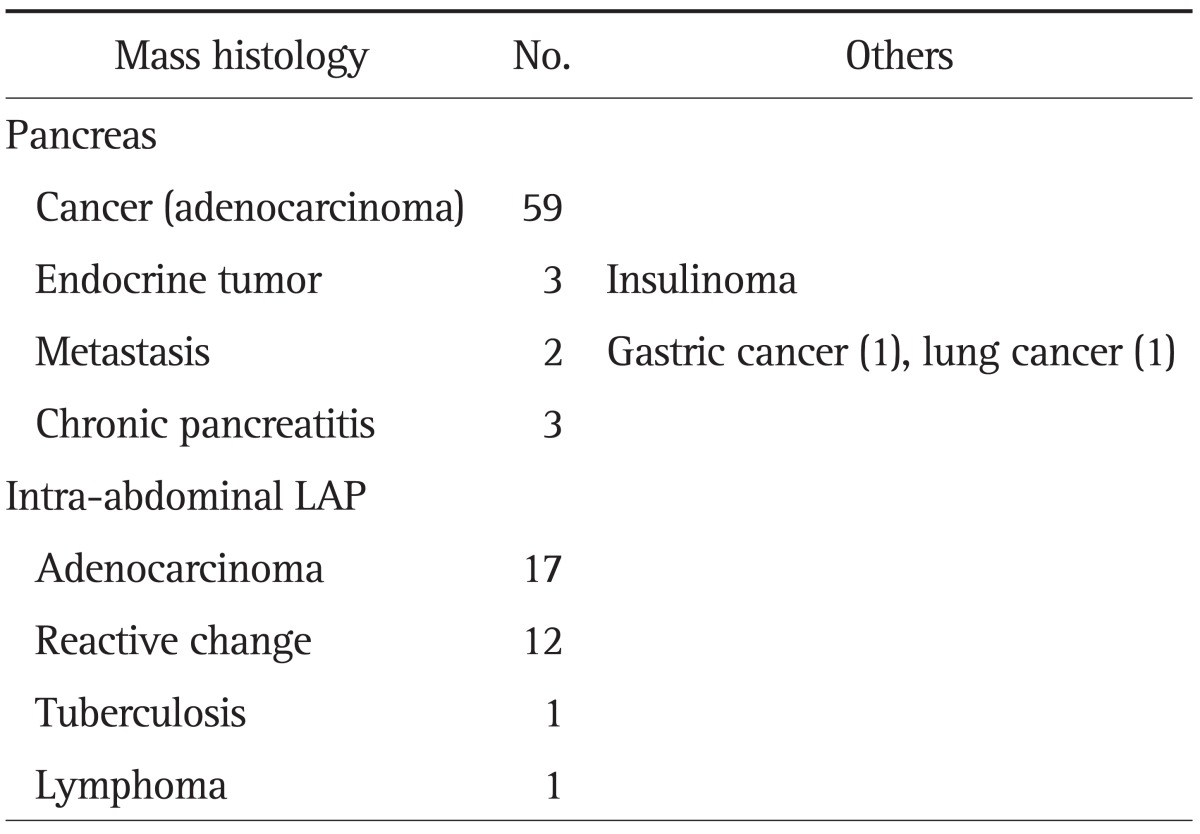
LAP, lymphadenopathy.
The percentage of cases in which adequate material from all sites was obtained for cytologic and histologic analysis is shown in Table 3. Histology and subsequent cytology provided adequate tissue samples in 65.3% (95% CI, 55.8% to 74.7%) and 91.8% of cases (95% CI, 90.5% to 99.3%; p<0.0001), respectively, with a combined adequacy of 94.8% (95% CI, 90.5% to 99.3%). The rate of acquisition of adequate material from all sites was significantly different between cytology and histology. From the pancreas (n=67), adequate samples for cytology and histology were obtained by FNA in 91.0% (95% CI, 84.2% to 97.9%) and 68.7% of cases (95% CI, 57.5% to 79.8%; p<0.0001), respectively, with a combined adequacy of 94.0% (95% CI, 88.4% to 99.7%). The histological yield of EUS-FNA in the pancreas was significantly more adequate in the body and tail than in the head (86.9% vs 59.1%, p=0.03, respectively), although the cytological yield in the pancreas was not significantly different according to the location of pancreas (p=0.06). In the cases of intra-abdominal lymphadenopathy, cytology and histology achieved adequate sampling in 93.5% (95% CI, 84.9% to 100%) and 58.0% of cases (95% CI, 40.7% to 75.4%; p<0.0001), respectively, with a combined adequacy of 96.8% (95% CI, 90.6% to 100%).
Table 3.
Yield of Adequate Tissue from Endoscopic Ultrasound-Guided Fine Needle Aspiration
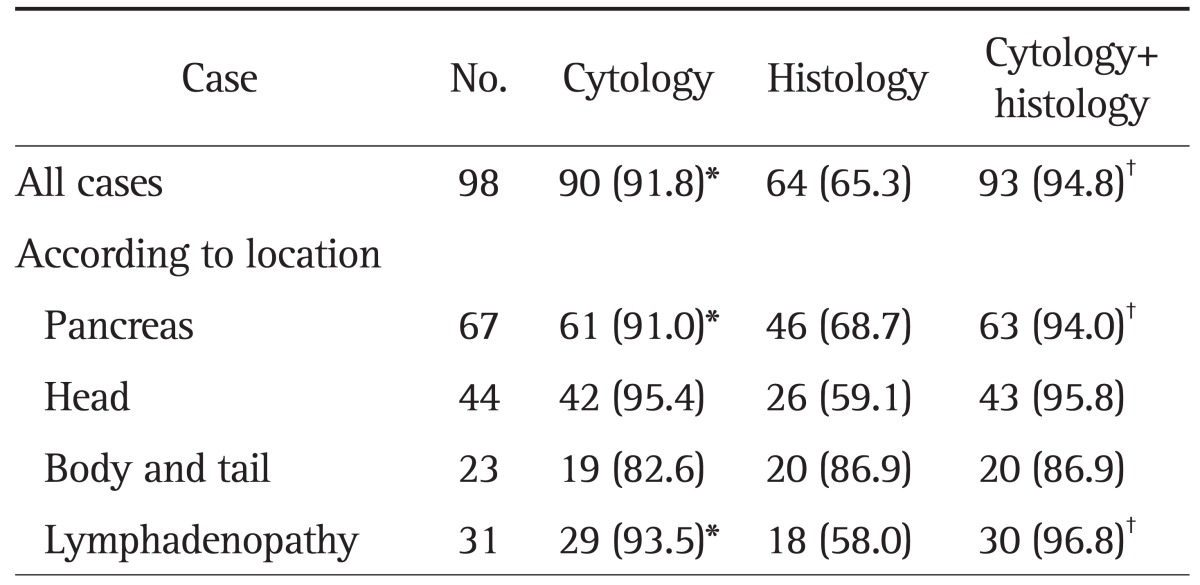
Data are presented as number (%).
*p<0.05 cytology vs histology; †p<0.05 histology vs cytology+histology.
The combined analysis of histology and cytology yielded the relative contribution of each test: sensitivity 84.1% (95% CI, 71.6% to 89.4%), specificity 100% and accuracy 86.7% (Table 4). Cytology alone had sensitivity 78.0% (95% CI, 63.1% to 82.8%), specificity 100% and accuracy 81.6%. The sensitivity and accuracy of the combined histology and cytology analysis was superior to those of cytology alone (78.0% and 81.6%), but was not significantly different (p=0.219). Histology alone had sensitivity 63.4% (95% CI, 43.6% to 64.8%), specificity 100% and accuracy 69.4%. The sensitivity and accuracy of the combined histology and cytology analysis was significantly higher than histology (p=0.006 and p=0.001, respectively). Of note, the histology analysis revealed five malignant lesions that were not diagnosed by cytology. There were no false-positive results from the cytology and histology analysis.
Table 4.
Diagnostic Discrimination of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology and Histology for All Cases
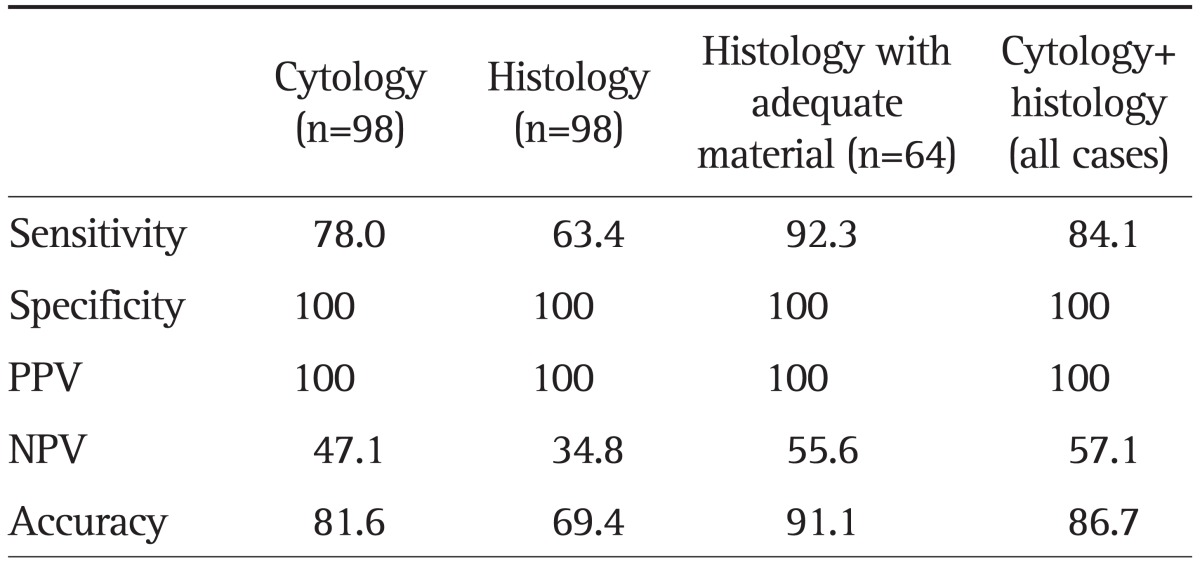
Data are presented as percentage. The combination of histology and cytology was significantly more accurate and sensitive than histology (p=0.006 and p=0.001) but was not significantly different from cytology in accuracy or sensitivity.
PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
EUS-FNA is a well-established technique for diagnosing lesions within the GI lumen or in organs or lymph nodes located adjacent to the esophagus, stomach, and duodenum. One of the current aims of EUS research is to define techniques that will maximize accuracy while minimizing cost. More recently, the EUS-TCB tissue acquisition method has been introduced as a means to obtain core biopsies for the evaluation of suspected malignancies, including pancreatic lesions. The advantages of needle core biopsy include the greater familiarity of the histological preparation procedures and preservation of the tissue architecture, which may be important in the assessment and subtyping of some tumors.5,6 In line with these considerations, we attempted to obtain histologic information from EUS-FNA specimens by using a standard 22- or 25-gauge FNA needle. The rationale for adding core biopsy to cytology by conventional FNA in our study was to maximize tissue adequacy when no on-site cytopathologist was present, as it has been reported that EUS-FNA in the absence of an on-site reviewer can result in a 10% to 15% reduction in the rate of definitive cytologic diagnosis.7
Adequacy assessment is an interesting debatable issue and no criteria for this assessment have been suggested. In our experience, the main benefit of the combined cytology and histology analysis by FNA is in the adequacy of the material that is obtained (94.8%). The overall cytological yield of EUS-FNA was more adequate than the histological yield for all lesions (91.8% vs 65.3%, respectively). The cytological yield of EUS-FNA without an on-site cytopathologist was considered adequate in 91.0% of the pancreatic masses and in 93.5% of the intra-abdominal lymph nodes, thus providing evidence that EUS-FNA is an effective diagnostic tool for obtaining adequate cytological samples. This is similar to the previously reported EUS-FNA sensitivity of 80% to 90% for pancreatic cancer.8-10
EUS-FNA with core biopsy may be effective in achieving adequate material acquisition. However, only a few studies have compared cytology to histology undertaken by EUS-FNA while including pancreatic masses.11,12 Binmoeller et al.11 reported that the histological yield of an 18-gauge FNA needle was less (68%) than the cytological yield (75%). Also Ito et al.13 showed that diagnostic accuracy of histology obtained by EUS-FNA without on-site cytopathologist was 90.7% by 19-, 22-, 25-gauge needle in pancreatic solid masses. In present study, the histological yield of EUS-FNA with 22- or 25-gauge needle was 65.3%, and it was more adequate in the pancreas than in the intra-abdominal lymph nodes, but although not significantly (68.7% vs 58.0%, p=0.31, respectively). Gerke et al.14 reported that the histological yield of FNA with high suction (27.8%) was significantly lower than that of TCB (95.3%). The inclusion of lymph nodes in Gerke's study may partially explain the lower yield of histology samples by FNA.14 This result may be related to the histological characteristics of the lymph nodes, particularly in that they are very fragile and smooth. Several studies have suggested that FNA with reduced or no suction may increase cytological lymph node specimen yield, whereas specimens obtained using higher suction may become bloody.15,16 Furthermore, our study included 13 benign lesions (41.9%) among 31 patients with intra-abdominal lymph nodes; we could not obtain adequate tissue samples for 76.9% (n=10) of patients with benign lymph nodes by EUS-FNA with usual suction. Also in this study, the histological yield of EUS-FNA in the pancreas was significantly more adequate in the body and tail than in the head (86.9% vs 59.1%, p=0.03, respectively). This difference can be explained that the endoscope can be located less angulated for lesions of the body and tail than in the head, resulting in easily targeting to obtain sufficient material successfully.
The diagnostic efficacy of EUS-FNA for cytology may vary greatly depending on the treatment of the samples and the level of proficiency of the cytopathologist when only smear method is used. Solid pancreatic masses are the most common target of EUS-FNA for cytology, which is reported to have 73% to 90% sensitivity, 95% to 100% specificity, and 81% to 95% accuracy for these lesions.17-20 In the present study, cytology by FNA without an on-site cytopatholgical examination was more sensitive than histology by FNA (78.0% for cytology vs 63.4% for histology). Classification of cytology findings as positive in this study was limited to positive malignancy, neoplasm, or suspicious for malignancy with atypical cases being counted as negative. This classification may affect the result of this study.
A combination of cytology and histology analysis may be ideal, as these procedures can complement each other when used in conjunction. The study by Moller et al.12 showed significantly better accuracy (87.5%) and sensitivity (82.9%) rates for the combined histology and cytology analysis in pancreatic tumors than those of cytology alone (77.6% and 68.1%, respectively). Also in the present study, the combination of cytology and histology by conventional FNA were more accurate (86.7%) and sensitive (84.1%) than cytology alone (81.6% and 78.0%, respectively), although we did not observe a significant difference. Furthermore, histology by EUS may be superior to cytology for establishing some specific diagnoses, especially in cases of benign tumors or if immunohistochemical staining is required. Some endosonographers have tried to find better methods than only smear method. Noda et al.21 reported that the cell block method with only HE staining showed a higher diagnostic yield than smear cytology (sensitivity 72%, accuracy 79%) who had undergone EUS-FNA without an on-site cytopathologist. The method for making cell block is a little complex.
The safety of EUS-FNA is well established. Our study demonstrated that EUS-FNA had a similar safety profile, with no significant complications occurring in patients who underwent both sampling modalities. However, the use of this combination method may result in more needle passes per case and a higher cost. Only one minor complication (self-limited mild pancreatitis) out of 95 patients (1%) was observed. The complication rate of EUS-FNA alone has been reported to be approximately 0.5%.1 The complications that were reported in a large TCB series occurred in 2% of cases, which included bronchopneumonia, minor hemoptysis, minor hematemesis, mucosal tear, and retroperitoneal abscess.20
EUS-FNA is associated with high diagnostic accuracy if an experienced cytopathologist is present in the endoscopy unit during the procedure and confirms the adequacy of the material using a rapid smear cytology technique (such as Diff-Quick stain) immediately after tissue acquisition.22,23 However, due to the financial constraints of most Korean institutions, the on-site presence of a cytopathologist for every EUS-FNA is unlikely in practice. This study revealed that the histology and cytology combination method by conventional FNA might increase the accuracy of EUS tissue sampling when no cytopathologist is present. This approach is promising for clinical use and has the potential to eliminate the need for TCB for establishing a diagnosis.
However, there are some limitations in the design of our study. The present study was a single-center retrospective review of a relatively small number of patients. Second, the final diagnosis was not confirmed by histological examination of the resected specimens in most patients. Further studies such as randomized, controlled trials with an adequate number of patients are warranted to evaluate the necessity of confirmation by smear cytology with histology assessments.
In conclusion, the combination of smear cytology and histology analysis may increase the diagnostic yield in patients with pancreatic masses and intra-abdominal lymphadenopathy undergoing EUS-FNA without an on-site cytopathologist. This straightforward technique of core biopsy by EUS-FNA is an attractive alternative method for obtaining histologic core samples in areas where TCB is not available.
ACKNOWLEDGEMENTS
This paper was supported by Wonkwang University in 2012.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231–236. doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- 2.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 3.Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol. 2003;120:351–367. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 4.Saftoiu A, Vilmann P, Guldhammer Skov B, Georgescu CV. Endoscopic ultrasound (EUS)-guided Trucut biopsy adds significant information to EUS-guided fine-needle aspiration in selected patients: a prospective study. Scand J Gastroenterol. 2007;42:117–125. doi: 10.1080/00365520600789800. [DOI] [PubMed] [Google Scholar]
- 5.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 6.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 7.Eloubeidi MA, Cohn M, Cerfolio RJ, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: the value of demonstrating detached ciliary tufts in cyst fluid. Cancer. 2004;102:253–258. doi: 10.1002/cncr.20369. [DOI] [PubMed] [Google Scholar]
- 8.Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459–464. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 9.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844–850. doi: 10.1111/j.1572-0241.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 11.Binmoeller KF, Thul R, Rathod V, et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–127. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 12.Moller K, Papanikolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–69. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: a single-center experience. Dig Endosc. 2011;23(Suppl 1):34–38. doi: 10.1111/j.1443-1661.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerke H, Rizk MK, Vanderheyden AD, Jensen CS. Randomized study comparing endoscopic ultrasound-guided Trucut biopsy and fine needle aspiration with high suction. Cytopathology. 2010;21:44–51. doi: 10.1111/j.1365-2303.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhutani MS, Suryaprasad S, Moezzi J, Seabrook D. Improved technique for performing endoscopic ultrasound guided fine needle aspiration of lymph nodes. Endoscopy. 1999;31:550–553. doi: 10.1055/s-1999-125. [DOI] [PubMed] [Google Scholar]
- 16.Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–447. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 17.Ardengh JC, Lopes CV, de Lima LF, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112–3116. doi: 10.3748/wjg.v13.i22.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuhashi T, Ghafari S, Chang CY, Gu M. Endoscopic ultrasound-guided fine needle aspiration of the pancreas: cytomorphological evaluation with emphasis on adequacy assessment, diagnostic criteria and contamination from the gastrointestinal tract. Cytopathology. 2006;17:34–41. doi: 10.1111/j.1365-2303.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 20.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 21.Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010;45:868–875. doi: 10.1007/s00535-010-0217-5. [DOI] [PubMed] [Google Scholar]
- 22.Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 23.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]


