Abstract
OBJECTIVE:
The objective of this study was to assess the effects of resistance training on oxidative stress markers in the livers of ovariectomized rats.
METHOD:
Adult Sprague-Dawley rats were divided into the following four groups (n = 8 per group): sham-operated sedentary, ovariectomized sedentary, sham-operated resistance training, and ovariectomized resistance training. During the resistance training period, the animals climbed a 1.1-m vertical ladder with weights attached to their tails; the sessions were conducted 3 times per week, with 4-9 climbs and 8-12 dynamic movements per climb. The oxidative stress was assessed by measuring the levels of reduced glutathione and oxidized glutathione, the enzymatic activity of catalase and superoxide dismutase, lipid peroxidation, vitamin E concentrations, and the gene expression of glutathione peroxidase.
RESULTS:
The results showed significant reductions in the reduced glutathione/oxidized glutathione ratio (4.11±0.65 nmol/g tec), vitamin E concentration (55.36±11.11 nmol/g), and gene expression of glutathione peroxidase (0.49±0.16 arbitrary units) in the livers of ovariectomized rats compared with the livers of unovariectomized animals (5.71±0.71 nmol/g tec, 100.14±10.99 nmol/g, and 1.09±0.54 arbitrary units, respectively). Moreover, resistance training for 10 weeks was not able to reduce the oxidative stress in the livers of ovariectomized rats and induced negative changes in the hepatic anti-oxidative/oxidative balance.
CONCLUSION:
Our findings indicate that the resistance training program used in this study was not able to attenuate the hepatic oxidative damage caused by ovariectomy and increased the hepatic oxidative stress.
Keywords: Resistance Training, Oxidative Stress, Ovariectomy, Hepatic Steatosis
INTRODUCTION
Menopause (ovariectomy in animals) is associated with increased food intake and body weight, metabolic dysfunction, loss of bone mineral density, diabetes, impairment of muscle function, and increased inflammatory markers and oxidative stress (OS) (1-4). OS may result in lipid peroxidation of cell membranes, damage to proteins, and DNA and stellate cell activation. These processes in turn lead to fibrosis, chronic inflammation, and apoptosis in the liver (5). The measurement of multiple biomarkers, such as enzymatic and non-enzymatic antioxidants, lipid peroxidation, and cellular redox balance, is required to confirm the presence of oxidative stress in tissues. One of the most commonly measured markers of the cellular redox status is the reduced glutathione (GSH) and oxidized glutathione (GSSG) ratio (6,7).
Estrogen has anti-oxidative properties related to the A-ring phenolic hydroxyl group, which acts as an effective electron donor and a free radical scavenger and interrupts the lipoperoxidation reaction (8). Estrogen also protects females by up-regulating the expression of antioxidants such as glutathione peroxidase (GSH-Px) and manganese superoxide dismutase (MnSOD) (9,10). The influence of metabolic disturbances caused by ovariectomy on the liver is of clinical interest because these disturbances may aggravate liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), through the generation of reactive oxygen species (ROS) (11,12).
Nguyen et al. (13) demonstrated that women with breast cancer treated with tamoxifen, a non-steroidal anti-estrogenic drug, developed a higher level of intra-abdominal fat and increased incidence of hepatic diseases. Yasuda et al. (14) analyzed the effect of estradiol treatment on hepatic fibrosis in young male and female rats and found that estradiol treatment effectively promoted a dose-dependent suppression of hepatic fibrosis, with reduced collagen content and lower levels of protocollagen type I and II mRNAs. Nevertheless, hormone replacement therapy (HRT) is not universally accepted, mainly due to its contraindication in some patients, low compliance, fear of and aversion to the side effects, long-term risks of some types of cancer (15), and risk of myocardial infarction (16).
There is evidence that regular physical activity up-regulates the anti-oxidative defense system, attenuates the age-related increase in the concentration of cellular ROS in the rat liver, and confers protection against oxidative stress-associated diseases (17). However, the beneficial effects of exercise are lost upon exhaustion. Due to limited metabolic adaptation, strenuous exercise could generate increased levels of ROS and lead to a down-regulation of the antioxidative defense system (18).
Among the exercise interventions discussed in the literature, resistance training appears to be the most efficient modality in the attenuation of sarcopenia, osteopenia, hepatic steatosis, and body composition changes promoted by menopause and ovariectomy (19,20). Previous studies conducted by our group have shown that resistance training is able to decrease fat accumulation and deposition in the liver of ovariectomized (Ovx) rats (21) and to reduce the gene expression of molecules related to lypogenesis (22). Recent studies in humans have demonstrated that resistance training yields results similar to aerobic training with respect to the up-regulation of the antioxidative defense system, reduction in lipid peroxidation, and protection against oxidative damage based on serum analyses (23). However, there is evidence that high-intensity resistance exercise increases ROS production and causes oxidative damage (24). Among other organs, the liver is one of the most sensitive targets of exercise-induced oxidative stress (25), but the effect of resistance training on OS in the liver of ovariectomized rats remains unclear. Thus, the aim of this study was to examine the effect of ovariectomy and resistance training on OS biomarkers in the liver of ovariectomized rats. In view of the results described above, our hypothesis was that resistance training may attenuate the oxidative stress caused by ovariectomy in the rat liver.
METHODS
Animals
Thirty-two female Sprague–Dawley rats from the breeding colony of the State University of São Paulo (UNESP, Araraquara, SP, Brazil), with initial weights of approximately 220±12 g, were used in this study. The animals had free access to water and chow and were kept in collective cages (four rats per cage) at a controlled temperature of 22±2 °C with a 12-h light/12-h dark cycle (light from 1 a.m. to 1 p.m.). All of the animals were fed balanced food for laboratory rats and mice (Biobase®, Águas Frias, Brazil), and food intake was monitored daily during the experimental period.
This study was approved by the Committee of Experimental Animals of the Federal University of São Carlos (Protocol no. 008/2010). All animal procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (26).
Experimental groups
The rats were randomly distributed into the following four experimental groups (eight animals per group): (I) sham-operated sedentary (Sham-Sed), (II) ovariectomized sedentary (Ovx-Sed), (III) sham-operated resistance training (Sham-Rt), and (IV) ovariectomized resistance training (Ovx-Rt). The sedentary animals (Sham-Sed and Ovx-Sed) were kept in their cages throughout the entire experimental period without any type of exercise. The ovaries were removed from the Ovx animals (Ovx-Sed and Ovx-Rt). The trained animals (Sham-Rt and Ovx-Rt) underwent a 10-week resistance training program, which was initiated at the same time for each group and is described below.
Ovariectomy and sham operation
Ovariectomies and sham operations were performed when the rats reached a weight of 250 g, according to the technique described by Kalu (27). For the surgery, the rats were anesthetized with a mixture of ketamine-xylazine (61.5-7.6 mg/kg, intraperitoneally). The sham-operated rats underwent the surgical procedure, but the ovaries were not removed. The ovaries were removed from the Ovx animals. All animals that underwent surgical procedures had 3 weeks of recovery, and all animals were euthanized 92 days after the surgical procedure.
Resistance training protocol
During the 10 weeks of resistance training, climbing sessions were performed 3 times per week. Initially, the rats were adapted to the resistance training protocol, which required them to climb a vertical ladder (1.1×0.18 m, 2-cm grid, 80° incline) with weights attached to their tails. The size of the ladder required the animals to perform 8-12 movements per climb. The load apparatus was attached to the tail by wrapping the proximal portion of the tail with a self-adhesive foam strip. A Velcro strap was wrapped around the foam strip and fastened. With the load apparatus attached to the tail, each rat was placed at the bottom of the ladder and familiarized with climbing. If necessary, a stimulus was applied to the animal's tail with tweezers to initiate the movement. At the top of the ladder, the rats reached a housing chamber (20×20×20 cm), where they were allowed to rest for 120 s. This procedure was repeated until the rats voluntarily climbed the ladder three consecutive times without stimuli.
The first training session started three days after this familiarization and consisted of four to eight ladder climbs with progressively heavier loads. For the initial climb, each animal carried a load that was 75% of its body mass. Subsequently, weight increments of 30 g were added until the load did not allow the animals to climb the entire length of the ladder. The highest load successfully carried over the entire length of the ladder was considered the rat's maximal carrying capacity for that training session.
The next training sessions consisted of four ladder climbs with 65, 85, 95, and 100% of the rat's previous maximal carrying capacity, as determined in the previous session. During subsequent ladder climbs, an additional 30-g load was added until a new maximal carrying capacity was determined. The resistance training protocol was adapted from that of Hornberger and Farrar (28), according to the needs of the current investigation.
Euthanasia and preparation of tissue samples
The animals were euthanized by decapitation 48 h after the last resistance exercise session. Immediately post-mortem, the livers were removed, washed with saline solution, weighed, frozen in liquid nitrogen, and stored at 80 °C until analysis. The right lobe of the liver was used for all analyses. The liver tissue was homogenized in a phosphate buffer (0.1 mol/L, pH 7.4) using a Turrax dispenser (MA 102 MINI E – Marconi Model) and centrifuged for 15 min at 6,500 × g (3000 rpm). The supernatant was used for the measurement of thiobarbituric acid reactive substances (TBARS) and for catalase and superoxide dismutase (SOD) activity assays.
Reduced and oxidized glutathione levels
Reduced and oxidized glutathione levels were measured in the liver as previously described (29). The assay is based on the reaction of GSH with 5.5' dithio-bis-2-nitrobenzoic acid (DTNB, Sigma, cat. no. D-8130). This reaction produces 5' thio–2–nitrobenzoic acid (TNB), which has a maximal absorbance at 412 nm, and oxidized glutathione–TNB adducts (GS–TNB). The rate of TNB formation is proportional to the concentration of total glutathione in the sample.
Liver samples (100 mg/mL for total glutathione measurements and 200 mg/mL for oxidized glutathione measurements) were homogenized in ice-cold 5% metaphosphoric acid solution (Sigma-Aldrich 239275) and centrifuged at 3,000 × g and 4 °C for 10 min. The clear upper layer was kept at 0-4 °C for the assay. KPE buffer (0.1 M potassium phosphate buffer with 5 mM EDTA disodium salt, pH 7.5) was used as a diluent to stock solution of DTNB (0.67 mg/mL prepared in KPE) and GR (40 µL of GR [250 units/mL] diluted in 3mL of KPE). A total of 20 µL of each blank, standard, or experimental sample and 120 µL of a mixture of equal volumes of DTNB stock solution (60 µL) and GR stock solution (60 µL) was added to each well, and the reaction was allowed to continue for 30s. The absorbance was measured at 412 nm in a microplate reader (SpectraMax® M5 with SoftMax® Pro Data Acquisition & Analysis software, Molecular Devices, Sunnyvale, CA, USA) every 30 s for a total of 5 min after the addition of 60 μL of 0.67 mg/mL β-NADPH in KPE. The rate of 2-nitro-5-thiobenzoic acid formation was used to calculate the total glutathione concentration from a GSH standard curve. For the GSSG assay, 2 μL of 2-vinylpyridine (Sigma-Aldrich no. 13229-2) was added to the 200 mL of liver homogenate, and the reaction was allowed to continue for 2 h in the dark. Subsequently, 20 μL of the homogenate was added to each well as described above. The GSSG concentration was calculated as the rate of 2-nitro-5-thiobenzoic acid formation based on a GSSG standard curve. The reduced GSH was calculated as the total glutathione - GSSG values, and the results were expressed as mmol/g tissue. The intra-assay coefficient of variation for reduced and oxidized glutathione was 1.6%, and the sensitivity of the assay was 7.8 μM/g of tissue.
Vitamin E
Vitamin E (a-tocopherol) was measured by HLPC as described by Jordão (30). Liver tissue was deproteinized with ethanol and then extracted with hexane. The evaporated organic layer was reconstituted with the mobile phase and injected using a 4.6×25-cm C-18-type Shim-pack CLC-ODS column (Shimadzu, Kyoto, Japan) and a 4 mm X 1-cm precolumn at a flow rate of 2.0 mL/min-1, and the absorbance was read at 292 nm in a UV-Vis detector. The results were expressed as nmol/g tissue. The intra-assay coefficient of variation for the measurement of vitamin E was 4%, and the sensitivity of the assay was 0.18 μM/g tissue.
Catalase and superoxide dismutase activities
CAT activity was assessed by measuring the rate of hydrogen peroxide (10 mmol·L–1) absorbance at 240 nm (31), with the activity expressed as U/mg protein. The intra-assay coefficient of variation for the measurement of CAT activity was less than 32.5%, and the sensitivity was 0.2 units/mg protein. SOD activity was determined using a commercial EIA kit from Cayman Chemical® (706002), which uses the xanthine and hypoxanthine method, with absorbance read at 460 nm. The SOD activity was determined based on standards of known SOD concentrations (U/mL), and the activity was expressed as U/g tissue. The intra-assay coefficient of variation for the SOD assay was 7.2%, and the sensitivity was 0.025 units/g tissue.
Lipid peroxidation
The TBARS assay was adapted from that of Costa (32). The liver samples were mixed with 1 mL of a solution containing 15% (w/v) trichloroacetic acid, 0.38% (w/v) thiobarbituric acid, and 0.25 N HCl. The mixture was heated at 100 °C for 30 min. The TBARS concentration was measured by the absorbance at 535 nm, using 1, 1, 3, 3-tetramethoxypropane (Sigma-Aldrich) as an external standard, and the results were expressed as μmol/g protein. The intra-assay coefficient of variation for the measurement of TBARS was 16.9%, and the sensitivity was 0.055 μM/g protein.
Protein determination
Total proteins were measured using a commercially available kit (LABTEST®; Labtest Diagnóstica, Lagoa Santa, Minas Gerais, Brazil).
Isolation of RNA and quantitative real-time (RT) polymerase chain reaction (PCR)
Total RNA was extracted from frozen liver tissue using the Trizol reagent (Invitrogen Corporation, California, USA) according to the manufacturer's specifications. The integrity and quality of the purified RNA were analyzed using formaldehyde denaturing agarose gel electrophoresis and by measuring the A260-to-A280 ratio. For the removal of genomic DNA, total RNA (1 μg) from each sample was treated with deoxyribonuclease I (DNase I; Invitrogen Corporation, CA, USA) according to a standard protocol recommended by the manufacturer. The treated RNA was reverse-transcribed into cDNA using M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA). The quantitative RT-PCR employed 25 μL-volume reactions containing SYBR Green PCR Master Mix (Fermentas®), 20 ng of cDNA, and 0.5 μM of each primer. The gene-specific primers were purchased from Invitrogen Life Technologies, Inc., and are listed in Table 1. The thermal cycling program consisted of 10 min at 95 °C, followed by 40 cycles of 94 °C for 15 s, 57-61 °C for 30 s, and 72 °C for 60 s. Following PCR, the melting curve was analyzed to ensure that only one PCR product was amplified per reaction.
Table 1.
Body parameters.
| Sham-Sed | Ovx-Sed | Sham-Rt | Ovx-Rt | |
| Body weight (g) | 342.57±8.77 | 385.87±8.29* | 309.62±6.62*,† | 371.62±7.44*,†,‡ |
| Food intake (g/d) | 20.63±0.49 | 22.38±0.55* | 20,48±0.39*,† | 22.34±0.61*,†,‡ |
| Uterus weight (g) | 0.83±0.13 | 0.19±0.02* | 0.76±0.13† | 0.16±0.00*,‡ |
| Femur weight (g/100 g BW) | 0.26±0.00 | 0.22±0.00* | 0.28±0.00*,† | 0.24±0.00†,‡ |
Values are presented as the means±SDM; n = 8 rats per group. BW: body weight, *significantly different from the Sham-Operated Sedentary (Sham-Sed) group (p≤0.05), † significantly different from the Ovariectomized Sedentary (Ovx-Sed) group (p≤0.05), ‡ significantly different from the Sham-Operated Resistance Training (Sham-Rt) group (p≤0.05).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. The relative expression of the quantitative RT-PCR products was determined using the ΔΔCt method, which calculates the relative expression using the following equation: fold induction = 2-[ΔΔCt], where Ct = the threshold cycle (i.e., the cycle number at which the sample's relative fluorescence surpasses the background fluorescence) and ΔΔCt = [Ct gene of interest (unknown sample) – Ct GAPDH (unknown sample)] – [Ct gene of interest (calibrator sample) – Ct GAPDH (calibrator sample)] (33). The primers used in this study were as follows: GSH-Px Fwd: 5-GGGCAAGGTGCTGCTCATTG-3 and Rev: 5-AGAGCGGGTGAGCCTTCTCA-3. The results were normalized to the housekeeping gene GAPDH through the use of the following primers: Fwd: 5- GATGCTGGTGCTGAGTATGTCG-3 and Rev: 5- GTGGTGCAGGATGCATTGCTGA-3. The following primers are available on the NCBI website: GSH-Px (NM_10336022) and GAPDH (NM_017008.3). The intra-assay coefficient of variation for GSH-Px was 6.6%.
Statistical analysis
All data are presented as the means and (±) standard deviation of the mean (SDM). The normality and homoscedasticity of the data were first tested by the Shapiro-Wilk and Levene's tests, respectively. Two-way analysis of variance (ANOVA) was used to compare the variables of resistance training and ovariectomy. Fisher's post-hoc test was used in the event of a significant (p≤0.05) ratio. SPPS version 17.0 (Somers, NY, USA) software was used, and the sample size was determined using G*Power version 3.1.3 (Kiel, Germany) with the following settings: level of significance at p = 0.05, power (1–β) = 0.80, and a large effect size (f2 = 0.40). Based on these a priori calculations, the final sample size was n = 39. Due to methodological and logistical aspects, the sample size used in the study was 32, and the sample power was therefore reduced to 0.74.
RESULTS
Compared with the Sham-Sed rats, the Ovx rats were found to have higher body weights and higher daily food intakes (p≤0.05). The Ovx rats also had lower femur and uterus weights than the Sham-Sed rats (15%; p = 0.032 and 77%; p = 0.001, respectively). However, the Sham-Rt group exhibited a significantly higher femur weight compared with the Sham-Sed (8%; p = 0.035) and Ovx-Rt (17%; p = 0.036) groups. In addition, the Ovx-Rt group showed higher femur weights (9%; p = 0.028) than the Ovx-Sed group (Table 1).
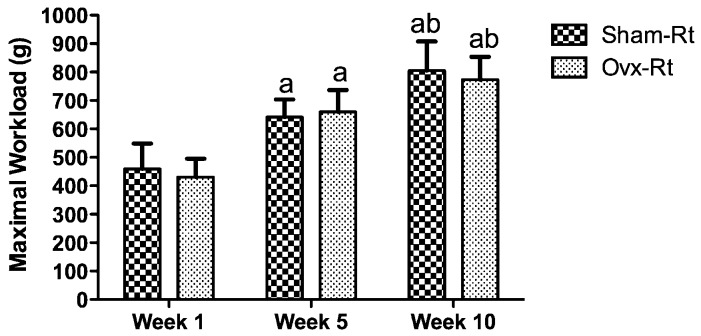
No group x time interaction was observed for the maximal workload during the 10-week training period, indicating that the maximal load carrying capacity increased in a similar manner for both the Sham-Rt and Ovx-Rt groups during the training period (Figure 1).
Figure 1.
The sham-operated resistance training (Sham-Rt) and ovariectomized resistance training (Ovx-Rt) groups' maximal workload at weeks 1, 5, and 10. Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to week 1, b statistically significant difference between weeks 5 and 10.
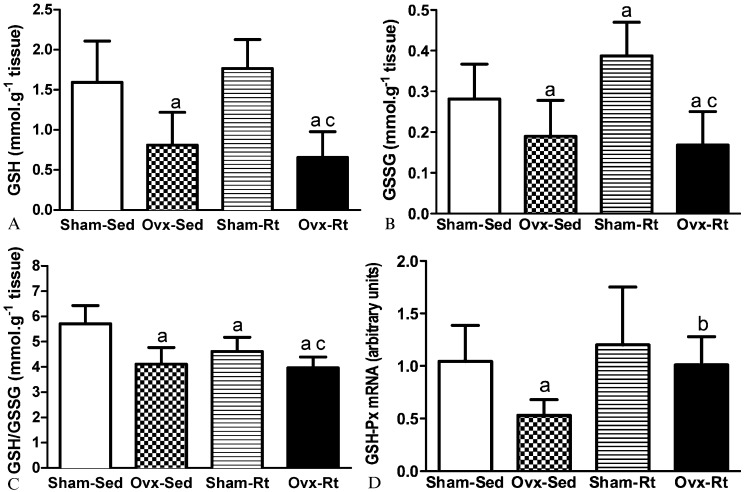
The GSH levels, GSSG levels, and GSH/GSSH ratios are shown in Figures 2A, B, and C, respectively. The Ovx-Sed group displayed lower GSH levels than the Sham-Sed group (49%, p≤0.0005; Figure 2A). Similarly, the Ovx-Rt group had lower GSH levels than the Sham-Sed group (62%, p≤0.005; Figure 2A).
Figure 2a - Levels of GSH (a) in liver homogenates 48h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to Sham-Sed, c compared to Sham-Rt.
Figure 2b - Levels of GSSG (b) in liver homogenates 48h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to Sham-Sed, c compared to Sham-Rt.
Figure 2c - Levels of GSH/GSSG (c) in liver homogenates 48h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to Sham-Sed, c compared to Sham-Rt.
Figure 2d - mRNA expression of glutathione peroxidase (GSH-Px) in the liver 48 h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to Sham-Sed, b compared to Ovx-Sed.
The GSSG levels were significantly higher in the Sham-Rt group than in the Sham-Sed group (38%, p = 0.021; Figure 2B), while the GSSG levels were significantly lower in the Ovx-Sed group than in the Sham-Sed group (32%, p = 0.032; Figure 2B). In addition, the Ovx-Rt group had lower GSSG levels than the Sham-Rt group (56%, p = 0.022; Figure 2B).
The GSSG levels were significantly lower in the Ovx-Sed and Ovx-Rt groups than in the Sham-Sed group (32%, p = 0.036 and 39%, p = 0.043, respectively) (Figure 2B). Furthermore, the GSSG levels were significantly higher in the Sham-Rt group compared with the other groups (p≤0.05; Figure 2B).
The GSH/GSSG ratio was lower in the Ovx-Sed and Sham-Rt groups than in the Sham-Sed group (28%, p≤0.005 and 19%, p = 0.001, respectively; Figure 2C). The GSH/GSSG ratio was lower in the Ovx-Rt group than in the Sham-Rt group (14%, p = 0.28; Figure 2C).
The RT-PCR results demonstrated lower gene expression of GSH-Px in the Ovx-Sed group compared with the Sham-Sed group (49%, p = 0.032; Figure 2D). In contrast, the gene expression of GSH-Px was higher in the Ovx-Rt group than in the Ovx-Sed group (90%; p = 0.043; Figure 2D).
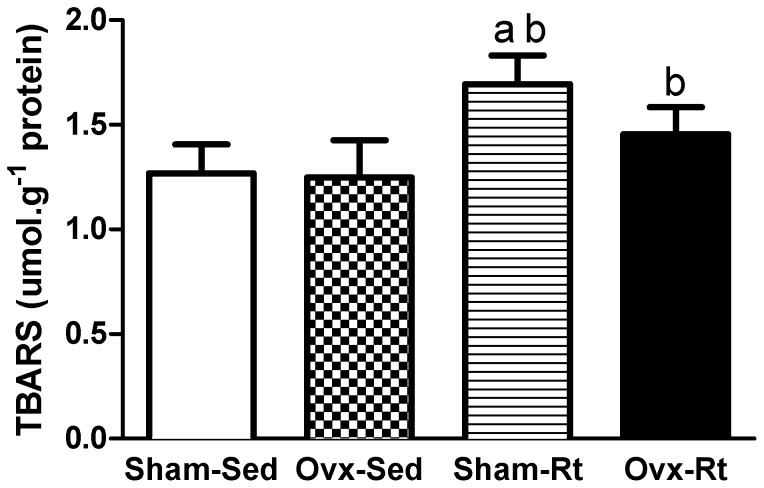
The Sham-Rt group was found to have a higher mean concentration of the lipid peroxidation marker TBARS than the Sham-Sed group (30%; p≤0.0005; Figure 3), while higher levels of TBARS were observed in the Ovx-Rt group compared with the Ovx-Sed group (23%, p = 0.004; Figure 3). However, no statistically significant differences in the concentrations of TBARS were observed between the Ovx-Sed and Sham-Sed groups.
Figure 3.
Lipoperoxidation levels in the liver homogenates 48h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt) groups. Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to Sham-Sed, b compared to Ovx-Sed.
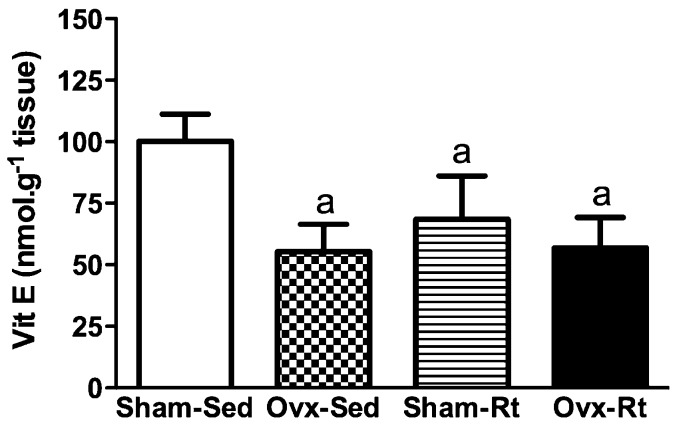
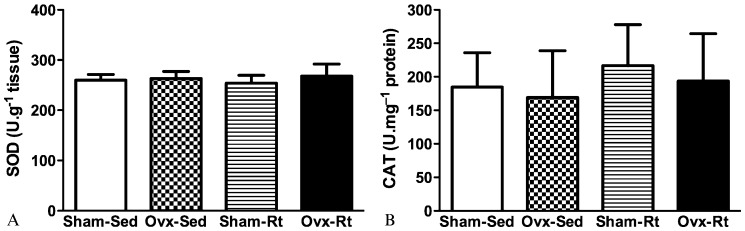
The concentration of vitamin E was found to be lower in the Ovx-Sed, Sham-Rt, and Ovx-Rt groups compared with the Sham-Sed group (45%, p≤0.0005; 35%, p≤0.0005 and 41%, p≤0.0005, respectively; Figure 4). No statistically significant differences in enzymatic SOD and CAT activity were observed between the groups (Figures 5A and B).
Figure 4.
Levels of Vitamin E in liver homogenates 48h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05. a statistically significant difference compared to Sham-Sed.
Figure 5a - Superoxide dismutase (SOD) (a) activity in the liver 48 h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05.
Figure 5b - Catalase (CAT) (b) activity in the liver 48 h after the last session of the resistance training program in sham-operated sedentary (Sham-Sed), ovariectomized sedentary (Ovx-Sed), sham-operated resistance training, (Sham-Rt) and ovariectomized resistance training (Ovx-Rt). Values are presented as mean ± SDM; p ≤ 0.05.
DISCUSSION
Regular exercise induces adaptations such as the hepatic down-regulation of ROS production (34,35). However, the beneficial effects of exercise are lost with exhaustion (18,36). In the present study, resistance training did not reverse the hepatic oxidative stress promoted by ovariectomy. Additionally, this resistance training protocol induced negative changes in the hepatic anti-oxidative/oxidative balance, as evidenced by the increased levels of TBARS (30%), decreased GSH/GSSG ratios (19%), and decreased vitamin E concentrations (35%).
Oxidative stress is an important mechanism involved in the progression of several ovariectomy-related pathogeneses, such as NAFLD and NASH (10,37). Regular physical exercise can promote antioxidative defenses and reduce OS (23,34). Radak et al. (17) showed that regular treadmill running for 8 weeks resulted in a 40% increase in the maximal oxygen uptake and caused a down-regulation of ROS production in the liver of trained rats. However, Ogonovszky et al. (18) demonstrated that the overtraining model, consisting of 1 hour of swimming/day for 5 days/week for 6 weeks, promoted oxidative damage to the hepatic nuclear DNA without influencing lipids or proteins. In that study, oxidative damage to the nuclear DNA was measured by 8-hydroxydeoxyguanosine (8-OHdG).
Recent studies have indicated that resistance training is beneficial for skeletal muscle, bones, and the liver (3,20-22). However, limited data are available regarding oxidative stress in the liver after this type of training. Margonis et al. (24) used a resistance training protocol with progressively increased and decreased volume/intensity in humans and demonstrated that overtraining induced a marked increase in oxidative stress biomarkers, which, in some cases, was proportional to the training load. Although the magnitude of the adaptation appears to be partly dependent on exercise intensity in mice and humans (24,38), a recent study with human subjects indicated that resistance training performed at intensities corresponding to hypertrophy and strength training yielded protective effects against OS similar to those provided by aerobic exercises, and these effects did not seem to be dependent on the training intensity (23).
Our study demonstrated that the ovariectomy procedure decreased the hepatic GSH levels in the sedentary and trained groups (Figure 2A). Glutathione is an antioxidant that protects mammalian cells against oxidative processes, and the liver is the largest producer and exporter of this antioxidant (39). The relationship between the two glutathione isoforms (GSH and GSSG) is considered an index of the cellular redox state; under increased OS conditions, the GSH/GSSG ratio decreases (39). In our study, resistance training and ovariectomy promoted a strong alteration of the liver redox state, as evidenced by the decreased GSH/GSSG ratio (Figure 2C).
Furthermore, ovariectomy markedly decreased the mRNA levels of GSH-Px (Figure 2D). Estradiol appears to up-regulate the expression of Mn-SOD and GSH-Px by interacting with the membrane estrogen receptor and activating MAP kinases and NF-kappaB in the mammary gland tumor cell line MCF-7 (56). However, resistance training also led to an increase in the GSH-Px mRNA levels (Figure 2D), corroborating the results of Chang et al. (57), who observed similar effects with endurance exercise.
Some types of training, such as moderate-intensity training, have been shown to provide protection against oxidative stress by increasing the hepatic gene expression and activity of Mn-SOD and GPx and by increasing the GSH levels (17,58). However, the increase in hepatic GSH-Px gene expression induced by our resistance training protocol was not accompanied by increased GSH levels, which are necessary to maintain the optimal catalytic condition of GSH-Px.
The literature has clearly demonstrated that changes in the glutathione levels caused by ovariectomy are accompanied by increased lipid peroxidation and increased MDA production (23). However, the ovariectomy procedure was not found to promote an increase in the MDA levels in this study (Figure 3). This result could be explained by the fact that the liver has a high mitotic potential and increased capacity for DNA repair (40).
Resistance training did increase the concentration of TBARS, regardless of the ovarian status (Figure 3). These results demonstrated that the resistance training protocol used in our study induced an oxidative process in the liver of these animals. The conversion of xanthine dehydrogenase (XD) into xanthine oxidase (XO) is among the mechanisms involved in the induction of oxidative damage in the liver during exercise. XO reduces O2 instead of NADP+, thereby producing superoxide ions (O2•) (4). Da Silva et al. (36) investigated the effects of different physical exercise protocols on OS markers in murine livers and found that continuous running and downhill running for 45 min daily, 5 days/week for 8 weeks, decreased the lipid peroxidation levels, as assessed by TBARS. This result demonstrated that the liver did not suffer damage from localized eccentric contractions. In addition, Rosety-Rodriguez et al. (34) reported that an 8-week moderate swimming training program reduced oxidative damage induced by emotional stress.
Oxidative stress and hepatic damage have been negatively correlated with vitamin E levels (42). Vitamin E is a fat-soluble antioxidant in the plasma that is primarily stored in the liver and performs the function of eliminating peroxyl radicals (ROO) (43). Moreover, several studies have clearly shown that serum levels of vitamin E are significantly reduced in patients with NAFLD (42). Zamin Jr. et al. (44) found that vitamin E supplementation significantly reduced OS in the liver and concluded that vitamin E plays an important therapeutic role in preventing diseases such as NASH. In our study, the reduced vitamin E concentrations in both groups of ovariectomized animals (sedentary and trained) can be explained by the low concentration of GSH, which reduces dihydroascorbate into ascorbate and thereby ensures that vitamin E is recycled (Figure 4). Furthermore, our resistance training protocol did not reverse the effects of ovariectomy and actually reduced the vitamin E concentrations in the trained sham group (Sham-Rt). A possible reason for the reduced vitamin E levels is the replenishment process, which involves the redistribution of vitamin E from the liver to the muscles during exercise (45). This result is in agreement with the findings of Aikawa et al. (46), who provided clear evidence of vitamin E depletion in the liver of trained rats under normal and reduced vitamin E diets.
Another physiological consequence of ovariectomy and menopause is an increase in CAT enzymatic activity and a decrease in the enzymatic activities of SOD and GSH-Px (47,48). These phenomena can be explained by the fact that when OS increases, SOD activity decreases, due to the irreversible inactivation of its H2O2 product. Meanwhile, the CAT activity increases to eliminate H2O2.
Although the results of previous training studies are sometimes conflicting, it is clear that regular physical activity leads to an increase in antioxidant enzyme activity, especially in tissues such as skeletal muscle (24,48). It has been established that strenuous physical exercise promotes oxidative stress due to the generation of oxygen free radicals, which stimulate the up-regulation of antioxidant enzyme activity in skeletal muscle. The effect of chronic exercise on OS in the liver and on antioxidative systems has been investigated in several studies, the results of which have shown that exercise training leads to reduced CAT activity (50,51) and increased SOD activity (52). This imbalance between SOD and CAT activity could be attributed to higher levels of pro-inflammatory molecules (53), which are known to increase SOD mRNA expression (54). In addition, CAT activity may be inhibited by the superoxide produced during exercise (55). In our study, no changes in SOD and CAT activity could be attributed to the physiological adaptation induced by OS (Figures 5A, B).
In summary, the relevant findings and limitations reported in this paper are as follows. The resistance training program used in this study failed to attenuate the hepatic oxidative damage caused by ovariectomy and increased the OS in the liver. One limitation of the present study was the lack of a group of ovariectomized animals treated with hormone replacement therapy to confirm the negative effects of resistance training on oxidative stress biomarkers in the liver. Further studies should be conducted to more accurately elucidate and characterize the psychological and metabolic stress imposed by this resistance training protocol in rats and humans.
ACKNOWLEDGMENTS
The authors thank Mr. José Carlos Lopes and Paula Payão for laboratory-related technical assistance. Financial support was provided by the Coordination for the Improvement of Higher Level (CAPES) and the Laboratory of Exercise Physiology Federal University of São Carlos (São Carlos, Brazil).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Signorelli SS, Neri S, Sciacchitano S, Pino LD, Costa MP, Marchese G, et al. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9(4):186–97. [PubMed] [Google Scholar]
- 3.Prestes J, de Cássia Marqueti R, Shiguemoto GE, Leite RD, Pereira GB, Selistre-de Araújo HS, et al. Effects of ovariectomy and resistance training on MMP-2 activity in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009;34(4):700–6. doi: 10.1139/H09-059. [DOI] [PubMed] [Google Scholar]
- 4.Prestes J, Leite RD, Pereira GB, Shiguemoto GE, Bernardes CF, Asano RY, et al. Resistance training and glycogen content in ovariectomized rats. Int. J. Sports. Med. 2012;33(7):550–4. doi: 10.1055/s-0032-1304646. [DOI] [PubMed] [Google Scholar]
- 5.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16(5):663–78. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52(8 Pt tio1):253–65. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 7.Powers SK, Jackson MJ. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol Rev. 2008 Oct. 88(4):1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niki E, Nakano M. Estrogens as antioxidants. Methods Enzymol. 1990;186:330–3. doi: 10.1016/0076-6879(90)86126-g. [DOI] [PubMed] [Google Scholar]
- 9.Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34(5):546–52. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 10.Vina J, Sastre J, Pallardo F, Borras C. Mitochondrial theory of aging: importance to explain why females live longer than males. Antioxid Redox Signal. 2003;5(5):549–56. doi: 10.1089/152308603770310194. [DOI] [PubMed] [Google Scholar]
- 11.Albano E, Mottaran E, Occhino G, Reale E, Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment Pharmacol Ther. 2005;22 Suppl 2:71–3. doi: 10.1111/j.1365-2036.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Grobe Y, Ponciano-Rodriguez G, Ramos MH, Uribe M, Mendez-Sanchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9(4):402–9. [PubMed] [Google Scholar]
- 13.Nguyen MC, Stewart RB, Banerji MA, Gordon DH, Kral JG. Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int J Obes Relat Metab Disord. 2001;25(2):296–8. doi: 10.1038/sj.ijo.0801488. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29(3):719–27. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- 15.Zhang SM, Manson JE, Rexrode KM, Cook NR, Buring JE, Lee I. Use of oral conjugated estrogen alone and risk of breast cancer. Am J Epidemiol. 2007;165(5):524–9. doi: 10.1093/aje/kwk038. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay C. Hormone replacement therapy is not what it used to be anymore. Rev Med Interne. 2004;25(11):806–15. doi: 10.1016/j.revmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, et al. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18(6):749–50. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 18.Ogonovszky H, Sasvari M, Dosek A, Berkes I, Kaneko T, Tahara S, et al. The effects of moderate, strenuous, and overtraining on oxidative stress markers and DNA repair in rat liver. Can J Appl Physiol. 2005;30(2):186–95. doi: 10.1139/h05-114. [DOI] [PubMed] [Google Scholar]
- 19.Pighon A, Gutkowska J, Jankowski M, Rabasa-Lhoret R, Lavoie JM. Exercise training in ovariectomized rats stimulates estrogenic-like effects on expression of genes involved in lipid accumulation and subclinical inflammation in liver. Metabolism. 2011;60(5):629–39. doi: 10.1016/j.metabol.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Shiguemoto GE, Prestes J, Leite RD, Pereira GB, Pontes CL, D'Avila FV, et al. Effects of resistance training on matrix metalloproteinase-2 activity and biomechanical and physical properties of bone in ovariectomized and intact rats. Scand J Med Sci Sports. 2012 Oct;22(5):607–17. doi: 10.1111/j.1600-0838.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 21.Leite RD, Prestes J, Bernardes CF, Shiguemoto GE, Pereira GB, Duarte JO, et al. Effects of ovariectomy and resistance training on lipid content in skeletal muscle, liver, and heart; fat depots; and lipid profile. Appl Physiol Nutr Metab. 2009;34(6):1079–86. doi: 10.1139/H09-116. [DOI] [PubMed] [Google Scholar]
- 22.Domingos MM, Rodrigues MF, Stotzer US, Bertucci DR, Souza MV, Marine DA, et al. Resistance training restores the gene expression of molecules related to fat oxidation and lipogenesis in the liver of ovariectomized rats. Eur J Appl Physiol. 2012;112(4):1437–44. doi: 10.1007/s00421-011-2098-6. [DOI] [PubMed] [Google Scholar]
- 23.Cakir-Atabek H, Demir S, PinarbaSili RD, Gunduz N. Effects of different resistance training intensity on indices of oxidative stress. J Strength Cond Res. 2010;24(9):2491–7. doi: 10.1519/JSC.0b013e3181ddb111. [DOI] [PubMed] [Google Scholar]
- 24.Margonis K, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Douroudos I, Chatzinikolaou A, et al. Oxidative stress biomarkers responses to physical overtraining:implications for diagnosis. Free Radic. Biol. Med. 2007;43(6):901–10. doi: 10.1016/j.freeradbiomed.2007.05.022. 15. [DOI] [PubMed] [Google Scholar]
- 25.Radak Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, Sasvari M, et al. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27(1-2):69–74. doi: 10.1016/s0891-5849(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 26.National Research Council. Washington, D.C.: National Academy Press; 1996. Guide for the care and use of laboratory animals; p. 140pp. [Google Scholar]
- 27.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15(3):175–91. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 28.Hornberger TA, Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16–31. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1(6):3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 30.Jordao AA, Jr, Chiarello PG, Arantes MR, Meirelles MS, Vannucchi H. Effect of an acute dose of ethanol on lipid peroxidation in rats: action of vitamin E. Food Chem Toxicol. 2004;42(3):459–64. doi: 10.1016/j.fct.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.Costa MC, Santos CCR, Lima SE. A simple automated precedure for thiol measurement in human serum samples. J Bras Patol Med Lab. 2006;42(5):345–50. [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Rosety-Rodriguez M, Ordonez FJ, Rosety I, Frias L, Rosety MA, Rosety JM, Rosety M. 8-weeks training program attenuates mitochondrial oxidative stress in the liver of emotionally stressed rats. Histol Histopathol. 2006;21(11):1167–70. doi: 10.14670/HH-21.1167. [DOI] [PubMed] [Google Scholar]
- 35.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44 (2):153–9. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Da Silva LA, Pinho CA, Rocha LG, Tuon T, Silveira PC, Pinho RA. Effect of different models of physical exercise on oxidative stress markers in mouse liver. Appl Physiol Nutr Metab. 2009;34(1):60–5. doi: 10.1139/H08-132. [DOI] [PubMed] [Google Scholar]
- 37.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412(15-16):1297–305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Vincent KR, Vincent HK, Braith RW, Lennon SL, Lowenthal DT. Resistance exercise training attenuates exercise-induced llipid peroxidation in the elderly. Eur J Appl Physiol. 2002;87(4-5):416–23. doi: 10.1007/s00421-002-0640-2. [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 40.Wei YH, Kao SH, Lee HC. Simultaneous increase of mitochondrial DNA deletions and lipid peroxidation in human aging. Ann N Y Acad Sci. 1996;786:24–43. doi: 10.1111/j.1749-6632.1996.tb39049.x. [DOI] [PubMed] [Google Scholar]
- 41.Radak Z, Asano K, Inoue M, Kizaki T, Oh-Ishi S, Suzuki K, et al. Superoxide dismutase derivative prevents oxidative damage in liver and kidney of rats induced by exhausting exercise. Eur J Appl Physiol Occup Physiol. 1996;72(3):189–94. doi: 10.1007/BF00838637. [DOI] [PubMed] [Google Scholar]
- 42.Leo MA, Rosman AS, Lieber CS. Differential depletion of carotenoids and tocopherol in liver disease. Hepatology. 1993;17(6):977–86. [PubMed] [Google Scholar]
- 43.Ostman B, Michaelsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, et al. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radic Biol Med. 2009;47(5):668–73. doi: 10.1016/j.freeradbiomed.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Zamin I, Jr, Mattos AA, Mattos AZ, Coral G, Santos D, Rhoden C. The vitamin E reduces liver lipoperoxidation and fibrosis in a model of nonalcoholic steatohepatitis. Arq Gastroenterol. 2010;47(1):86–92. doi: 10.1590/s0004-28032010000100015. [DOI] [PubMed] [Google Scholar]
- 45.Swift JN, Jr, Kehrer JP, Seiler KS, Starnes JW. Vitamin E concentration in rat skeletal muscle and liver after exercise. Int J Sport Nutr. 1998;8(2):105–12. doi: 10.1123/ijsn.8.2.105. [DOI] [PubMed] [Google Scholar]
- 46.Aikawa KM, Quintanilha AT, de Lumen BO, Brooks GA, Packer L. Exercise endurance-training alters vitamin E tissue levels and red-blood-cell hemolysis in rodents. Biosci Rep. 1984;4(3):253–7. doi: 10.1007/BF01119661. [DOI] [PubMed] [Google Scholar]
- 47.Tresguerres JA, Kireev R, Tresguerres AF, Borras C, Vara E, Ariznavarreta C. Molecular mechanisms involved in the hormonal prevention of aging in the rat. J Steroid Biochem Mol Biol. 2008;108(3-5):318–26. doi: 10.1016/j.jsbmb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Trevisan M, Browne R, Ram M, Muti P, Freudenheim J, Carosella AM, et al. Correlates of markers of oxidative status in the general population. Am J Epidemiol. 2001;154(4):348–56. doi: 10.1093/aje/154.4.348. [DOI] [PubMed] [Google Scholar]
- 49.Terblanche SE. The effects of exhaustive exercise on the activity levels of catalase in various tissues of male and female rats. Cell Biol Int. 2000;23(11):749–53. doi: 10.1006/cbir.1999.0442. [DOI] [PubMed] [Google Scholar]
- 50.Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol. 1990;68(6):2337–43. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- 51.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272(1 Pt 2):R363–9. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 52.Burneiko RC, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GM, Faine LA, et al. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006;44(7):1167–72. doi: 10.1016/j.fct.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 54.Dougall WC, Nick HS. Manganese superoxide dismutase: a hepatic acute phase protein regulated by interleukin-6 and glucocorticoids. Endocrinology. 1991;129(5):2376–84. doi: 10.1210/endo-129-5-2376. [DOI] [PubMed] [Google Scholar]
- 55.Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257(10):5751–4. [PubMed] [Google Scholar]
- 56.Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, et al. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4(3):113–8. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 57.Chang SP, Chen YH, Chang WC, Liu IM, Cheng JT. Increase of anti-oxidation by exercise in the liver of obese Zucker rats. Clin Exp Pharmacol Physiol. 2004;31(8):506–11. doi: 10.1111/j.1440-1681.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 58.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44 (2):126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]