Figure 4.
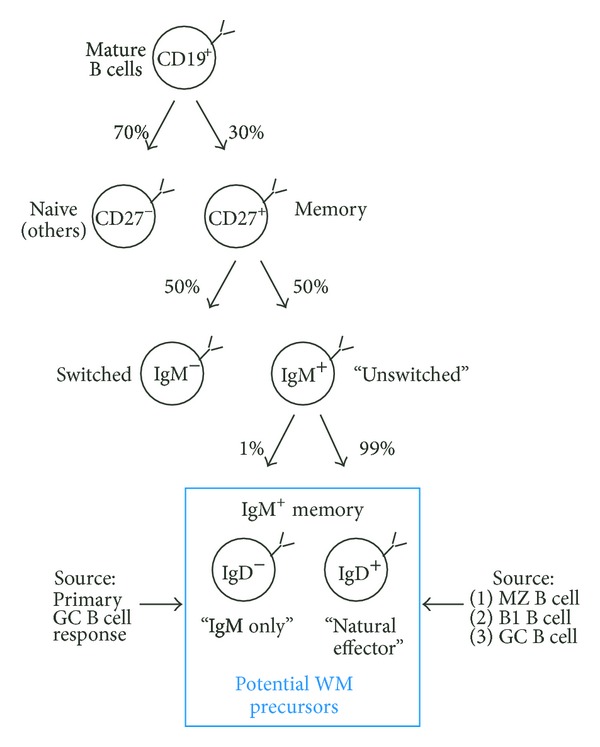
Possible origin of WM precursors from IgM+ memory cells. Of human splenic and peripheral blood B cells, ~70% are CD27− and ~30% are CD27+. The CD27− cells comprise a mixed population that contains both naive IgM+ B cells that express germ line IgV genes and IgG+/IgA+ memory B cells that harbor mutated IgV genes (not shown). Naive B cells have not yet participated in an immune response and not yet modified their expressed IgV genes using the somatic hypermutation (SHM) pathway. In contrast, memory B cells are immunologically experienced and have engaged the SHM machinery to increase the affinity of the expressed IgV gene to the underlying antigen. The CD27+ compartment is composed equally of “switched” IgM− B cells and “unswitched” IgM+IgD+ B cells. The former have performed H chain class switch recombination (CSR) and, thereby, replaced their μH with γ/α/εH chains, while the latter have not. The great majority of CD27+IgM+ cells coexpress IgD (IgM+IgD+)—a compartment that is often referred to as “natural effector” memory. CD27+IgM+IgD− B cells, sometimes called “IgM-only” cells for short, only represent a minor population (1%) in peripheral blood and spleen. Both CD27+IgM+IgD+ and CD27+IgM+IgD− memory B cells may be precursors of WM, although the weight of the evidence seems to favor the IgM+IgD+ compartment as the stronger candidate. This compartment likely constitutes a mixture of marginal zone (MZ)-, B1- and germinal-center- (GC-) derived B cells. The CD27+IgM+IgD− compartment is better defined as it originates from a single source: the primary GC response, which often selects for mutated IGKV3-20 alleles.
