Figure 5.
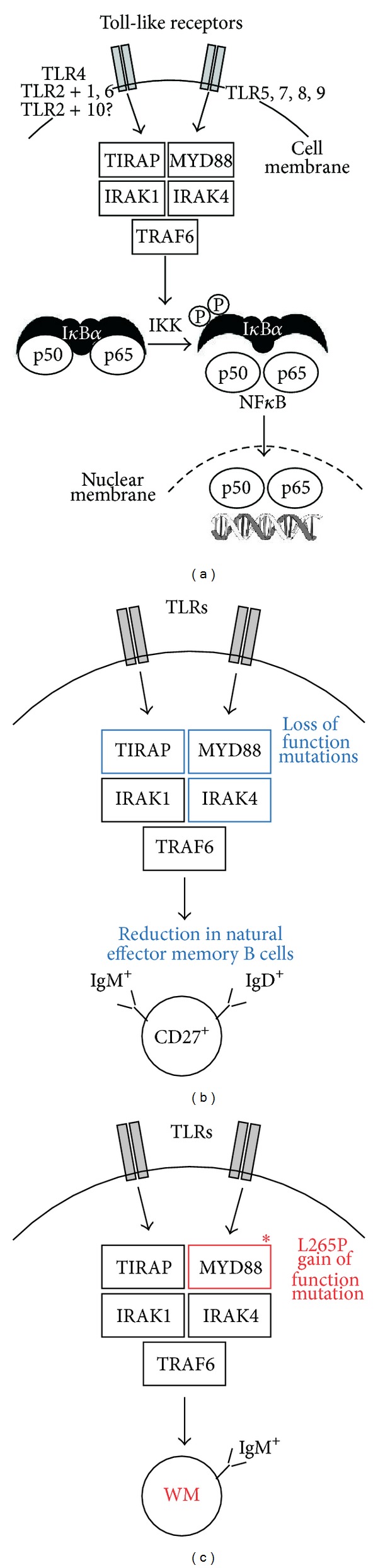
MYD88—an intriguing link between WM and natural effector IgM+ memory cells. (a) In normal B-lymphocytes and other cells of the immune system, MYD88 functions as a signaling adaptor protein that activates the nuclear factor κB (NF-κB) pathway following stimulation of Toll-like receptors (TLRs) and receptors for IL-1 and IL-18 (not shown). MYD88 coordinates the assembly of a supramolecular signaling complex that contains members of the IRAK (interleukin-1 receptor-associated kinase) family of serine-threonine kinases. Following TLR ligand binding, MYD88 is recruited to the cell membrane-bound receptor complex, leading to recruitment of IRAK4, which activates IRAK1 and IRAK2 (not shown) by phosphorylation of serine and threonine residues [177, 178]. IRAK1-dependent activation of tumor necrosis factor receptor-associated factor 6 (TRAF6) affects NF-κB activation via phosphorylation of IκBα and execution of the NF-κB-dependent gene regulation program. TIRAP is an additional adapter protein involved in TLR4 and TLR2 signaling (see Section 5.1 for details). (b) Genetic loss of MYD88, IRAK4, and TIRAP function leads to significant reductions in CD27+IgM+IgD+ memory B cells, indicating that the MYD88-TIRAP-IRAK4 pathway is essential for homeostatic maintenance of the natural effector memory. (c) WM cells harbor the highly recurrent, gain-of-function L265P exchange in the MYD88 adaptor protein, indicating that WM depends on elevated TLR signaling. This is intriguing in light of the findings depicted in (b) and suggests that WM is derived from natural effector memory B cells.
