Abstract
Ballast material (organic, opal, calcite, lithogenic) is suggested to affect sinking speed of aggregates in the ocean. Here, we tested this hypothesis by incubating appendicularians in suspensions of different algae or Saharan dust, and observing the sinking speed of the marine snow formed by their discarded houses. We show that calcite increases the sinking speeds of aggregates by ~100% and lithogenic material by ~150% while opal only has a minor effect. Furthermore the effect of ballast particle concentration was causing a 33 m d-1 increase in sinking speed for a 5×105 µm3 ml-1 increase in particle concentration, near independent on ballast type. We finally compare our observations to the literature and stress the need to generate aggregates similar to those in nature in order to get realistic estimates of the impact of ballast particles on sinking speeds.
Introduction
Sinking marine particles and especially marine snow are responsible for a significant fraction of the sinking carbon in the ocean [1,2] and thus play an important role in the biological carbon pump [3]. Important efforts have been made to understand how sinking aggregates are formed in surface layers, sink through the water column and are consumed during their path to the bottom (see reviews in [4,5,6]). In particular, sinking speed variations have been attributed to both changes in particle size [7-10], composition, and density [11-13]. The quantity of organic carbon transported to depth by sinking particles has been observed to be closely associated to the quantity of inorganic constituents of the particles [11,12,14]. This inorganic part is mostly composed of opal, calcite, and lithogenic materials originating from, respectively, diatoms, coccolithophores or foraminifers, and atmospheric or river inputs. These constituents play a ballasting role on the particles and increase their sinking velocity because of their high density [15-19]. However, in most studies, marine snow particles were obtained artificially under laboratory conditions using long incubations of dense algal culture in roller tanks, producing particles with characteristics different from those formed in situ [20]. Therefore, there is a strong need to confirm these observations with realistic aggregates.
Appendicularians are planktonic tunicates that use external mucous devices, called houses, to filter, concentrate, and feed on particles ranging in size from 0.2 µm to several hundreds of microns [21,22]. These houses can clog, thus forcing the appendicularian to discard the house and secrete a new one several times per day (up to 27 d-1 [23]). The discarded houses can include concentrated non-ingested particles such as bacteria, phytoplankton, and lithogenic dusts [17,24,25], while scavenging of additional particles by sinking houses has been shown to only play a minor role in their final composition [26]. These mucous feeding structures are an important source of marine snow formed by zooplankton [27], and may contribute significantly to POC flux [28,29]. Despite this potentially high contribution to carbon flux, only few studies have focused on appendicularians’ impact on POC fluxes [30], and nothing is known about the impact of food or ballasting particles (eg. [31]) on the sinking properties of discarded houses. Appendicularians are easy to cultivate [32,33], and because house production is a biologically controlled process, it allows the production of discarded houses in the laboratory with physical properties similar to those produced in the ocean. Moreover, appendicularian-produced marine snow has sinking characteristics comparable with phytoplankton aggregates [7,27,34], which makes these biological aggregates a good proxy for marine snow particles and suitable for testing the effect of ballast particle composition.
In this study we used the appendicularian Oikopleura dioica to produce houses loaded with different kind of ballasting material and tested the influence of different concentrations and types of ballasting material on the sinking speed.
Materials and Methods
Four types of marine snow particles were formed from appendicularian houses that had integrated four types of “ballasting” material. The ballasting particles were obtained through three species of phytoplankton and Saharan dust. The organic ballasting materials were Isochrysis galbana (flagellate algae), opal ballast was provided by Thalassosira pseudonana (silicifying diatom), and calcite ballast by Emiliana huxleyi (calcifying coccolithophorids). All phytoplankton were cultivated in F/2 medium (Guillard) with addition of selenium for E . huxleyi and under 12h: 12h light cycle (250 µE m‑2 s-1). The dust particles were collected and treated as described in [35]. The resulting marine snow particles will hereafter be referred to as organic, opal, calcite, and lithogenic particles.
Appendicularians ( Oikopleura dioica ) were collected in the Øresund strait, western Baltic Sea, and grown in the laboratory following standard protocols [34,36]. According to the Danish legislation on experimental animals, no permission is required to collect invertebrates (octopuses being the exception) in Danish National waters. Under laboratory conditions (16°C; 35 psu) O . dioica produces 4-6 houses d-1 [37] and then each house is used about 5 hours before being discarded.
In order to obtain sinking speeds of discarded houses loaded with the 4 different ballasting materials the experimental setup followed 3 steps(1). Appendicularians were first placed in a suspension of the ballasting material, thus producing discarded houses loaded with this material(2). Discarded houses were next allowed to age and deflate, and (3) their sinking speeds were finally measured. The same 0.2 µm-filtered seawater was used over the course of the experiment ensuring similar salinity and temperatures conditions.
For each type of ballasted particle we used three different concentrations of ballast particles, representative of a monospecific bloom or an intense Saharan dust event, to load the discarded houses: a medium concentration, representing meso- to eutrophic conditions (20 000 cells ml-1 for I . galbana and T. pseudonana), a lower (0.5 × medium), and a higher concentration (1.5 × medium). These concentrations correspond grossly to pre-bloom, beginning of bloom, and bloom conditions as recorded in coastal temperate environments [38]. The concentrations of Saharan dusts and E . huxleyi were obtained based on equivalent particles volume concentrations. Medium concentration of E . huxleyi (18 000 cells ml-1) corresponds to bloom condition [15] while medium Saharan dusts concentrations corresponded to 5-12 mg L-1, which is equivalent to a strong Saharan dust rain event [39]. Particle concentrations were measured using a coulter counter both at the beginning and the end of incubations, and mean concentration over the experiments were reported (Table 1).
Table 1. Type, initial, final and mean concentration of ballast particles used to produce appendicularian houses and recorded sinking properties of those houses.
| Type of particles and concentration used | Particulate volume concentration (µm3 ml-1) | n | Mean house size | Mean sinking speed | Mean excess density | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| initial | final | mean | (mm ± std) | (m d-1 ± std) | (mg cm-3 ± std) | ||||||
| Organic | 0.5 | 8.52 105 | 6.67 105 | 7.60 105 | 10 | 1.80 | ± 0.31 | 45.29 | ± 25.53 | 0.36 | ± 0.20 |
| 1 | 1.27 106 | 6.81 105 | 9.76 105 | 10 | 1.89 | ± 0.49 | 89.98 | ± 28.66 | 0.78 | ± 0.33 | |
| 1.5 | 1.61 106 | 1.38 106 | 1.50 106 | 10 | 2.61 | ± 0.46 | 75.90 | ± 34.09 | 0.31 | ± 0.10 | |
| Opal | 0.5 | 1.15 106 | 7.64 105 | 9.57 105 | 10 | 2.12 | ± 0.38 | 82.78 | ± 39.65 | 0.39 | ± 0.26 |
| 1 | 1.44 106 | 1.01 106 | 1.23 106 | 10 | 2.35 | ± 0.44 | 110.94 | ± 39.61 | 0.59 | ± 0.14 | |
| 1.5 | 1.87 106 | 1.44 106 | 1.66 106 | 10 | 2.37 | ± 0.37 | 125.20 | ± 30.44 | 0.68 | ± 0.13 | |
| Calcite | 0.5 | 6.47 105 | 5.21 105 | 5.84 105 | 10 | 1.97 | ± 0.48 | 108.05 | ± 38.98 | 0.88 | ± 0.37 |
| 1 | 1.15 106 | 8.51 105 | 1.00 106 | 10 | 1.98 | ± 0.43 | 141.22 | ± 47.95 | 1.02 | ± 0.59 | |
| 1.5 | 1.63 106 | 1.32 106 | 1.47 106 | 10 | 2.13 | ± 0.58 | 167.39 | ± 62.10 | 1.23 | ± 0.42 | |
| Lithogenic | 0.5 | 8.99 105 | 2.26 105 | 5.63 105 | 10 | 1.77 | ± 0.51 | 139.12 | ± 75.82 | 1.40 | ± 0.80 |
| 1 | 1.64 106 | 8.93 105 | 1.27 106 | 10 | 2.70 | ± 0.66 | 159.46 | ± 51.03 | 0.79 | ± 0.30 | |
| 1.5 | 1.94 106 | 7.53 105 | 1.35 106 | 10 | 1.79 | ± 0.48 | 230.72 | ± 53.27 | 2.58 | ± 0.85 | |
| Kruskall-Wallis test | X9,90 = 18.86 | X3,90 = 40.91 | X3,90 = 46.74 | ||||||||
| p = 0.026 | p < 0.001 | p < 0.001 | |||||||||
Kruskall-wallis test between the different conditions examined is also indicated.
For each condition, 40 5-day old appendicularians were picked from the mother culture and placed in a 5-L bucket filled with 0.2-µm filtered seawater to which was added the ballast particles at the targeted concentration. The buckets were then manually strongly stirred to force the appendicularians to discard their house and secrete a new one. Subsequently the buckets with appendicularians were stirred gently and continuously using internal paddles (8 rpm) for 5 hours, which is sufficient time for the animals to have houses nearly ready to be discarded [37]. Ten appendicularians with houses loaded with particles and ready to be discarded were then placed in petri dishes filled with the same water as used for the incubation until they discarded their houses. Visual observation with dissecting microscope confirmed that there were no aggregation of any ballast particles in the bottom of the bucket, suggesting that all were affected similarly by the different stirring.
The freshly discarded houses were transferred using a wide mouth pipette to a 250-mL closed bottle placed on a rotating table with a rotation speed assuring minimal contacts of the house with the bottles walls. These incubations were done using 0.2-µm filtered seawater, preventing any additional fixation of particles through scavenging [26]. The houses were left for 2 hours, which is a sufficient time to pass the strong initial deflation process [34], thus allowing comparable measurement of sinking speed between houses. Finally, houses were recovered from the bottle using a wide bore pipette and placed in a Plexiglas chamber (dimensions 5x5x20 cm) illuminated with a red laser sheet. Here their sinking speed and size were recorded using video observations, and their excess density was calculated using the Newton-Rittinger equation as described in [34].
Results
The median size of appendicularian houses (2.11 mm, Table 1) was not strongly significantly different between experimental conditions (Kruskall-Wallis test). Therefore, the sinking speed measurements obtained between conditions can be directly compared, even if the house size variability may have increased the overall variability of sinking speed measurements (Table 1)
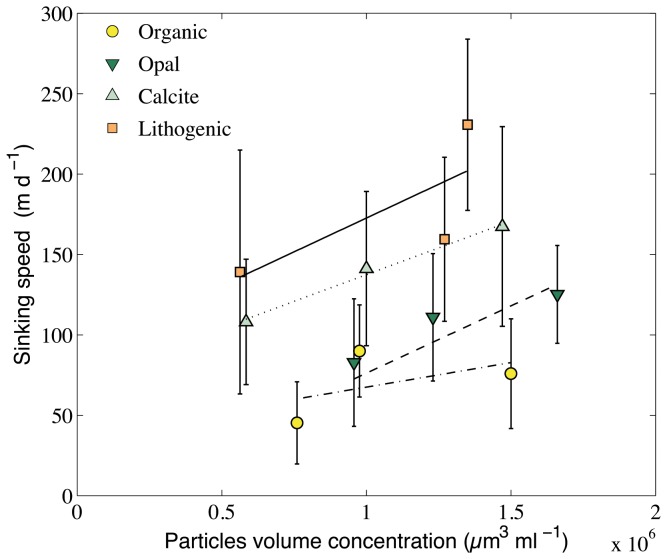
Sinking speeds differed significantly between the differently ballasted particles (Kruskall-Wallis test; Table 1) even if the sinking speed recorded between organic ( I . galbana ) and opal particles (T. pseudonana) were not significantly different (pairwise comparison test). Houses loaded with organic and opal material settled with a mean sinking speed of 88 m d-1, while houses loaded with calcite ( E . huxleyi ) and lithogenic particles sank, respectively, at average speeds of 138 and 176 m d-1. Because particles concentrations changed during the course of the experiments due to appendicularian feeding activity (Table 1), mean concentration may better represents the overall concentration during the experiment; however, even using initial or final concentration do not change qualitatively our results. The aggregates’ load concentration had an effect on the sinking speed with higher concentration of ballasted particles in the seawater promoting a higher sinking speed (Figure 1; all relationships except for organic particles are significantly different from zero) with a nearly constant effect of 33 m d-1 settling speed increase for a 5×105 µm3 ml-1 increase in particle concentration. Generally, calcite increased the sinking speeds of organic particles by ~100% and lithogenic material increases it by ~150%.
Figure 1. Effect of concentration and type of ballast particles on appendicularian houses sinking speed.
Concentrations of ballast particles correspond to concentration in the seawater in which the appendicularians were incubated and houses produced. Mean and standard deviations for each observation (n=10). Lines and dashed lines do represent the relationships between particles volume concentration (V) and sinking speed (S) with S = 3.04 ×10-5 V + 37.17 for organic particles; S = 8.38 ×10-5 V -7.82 for opal particles; S = 6.67 ×10-5 V + 70.82 for calcite particles; and S = 8.38 ×10-5 V + 88.63 for calcite particles. The slopes of all relationships were not significantly different (mean slope of 6.65×10-5 m d-1 µm-3 ml; covariance analysis, F3,111 = 0.75, p = 0.52), to the contrary of the intercepts (covariance analysis, F3,114 = 28.9, p < 0.001) to the exception of organic and opal ballasted particles (covariance analysis, F1,58 = 3.16, p = 0.08).
Similarly, the excess density calculated from the size of the appendicularian houses and their sinking speed (Table 1) was significantly different between the differently ballasted aggregates with the exception of organic compared to opal and calcite compared to lithogenic particles (pairwise comparison tests). Overall, organic particles and particles ballasted with opal were characterized by an excess density of 0.52 while calcite and lithogenic particles had an excess density of 1.32.
Discussion
This is the first study to test simultaneously the impact of different ballasting material and their concentrations on the sinking speed of biogenic aggregates such as appendicularian houses. To our knowledge, only few experiment have been performed in a similar set-up to examine the impact of different minerals concentrations on aggregates size, weight, density and composition [40-42], and the effect on sinking speed was only tested in one case [41]. We first show that the concentration of ballasting material in seawater has a significant effect on the sinking velocity of aggregates produced by appendicularians. Appendicularians only ingest a minor proportion of the filtered particles [43] and discard the non-ingested particles together with the clogged houses [23]. This mechanism leads to a load of ballast into the house that is proportional to the particle concentration in the surrounding seawater [26], thereby correlating ballast concentration and house sinking speed.
The present study provides new observations on the impact of four different ballasting materials on sinking speed of marine aggregates produced by appendicularians. This is of primary importance since sinking speed is one of the major parameter controlling carbon sequestration in the ocean. Our experiment relies on the use of laboratory-made appendicularians houses, which can be a major source of marine snow in the ocean [27]. Laboratory-made aggregates made this way are likely to produce aggregates comparable to those in situ [34] leading to realistic estimates of sinking speeds and excess densities. Our estimates were mostly based on monospecific diets while, in the field, appendicularians may often feed on a more diverse community including organic, opal, calcite and lithogenic based particles. However, our observation may replicate monospecific blooms or intense Saharan dust rain event where one type of particles is dominating the assemblage. Our estimates are similar to those obtained for aggregates formed from natural seawater or algal cultures [9,17,44], and from in situ observed aggregates [7,18](Table 2). However, both our sinking speed and excess density estimates are lower than some observations using E . huxleyi cultures as the only source of particles formation [15,16,42] or different minerals in combination with diatom cultures [41] for which both sinking velocities or excess densities seem high (Table 2). This suggests that some aggregates produced artificially in laboratory from cultures of algae may be different from natural aggregates. The exact reason remains unclear since the methodology (using “old” algae cultures in rotating tanks) is similar between those two groups of studies and therefore should get the same faster remineralization of organic fraction compared to inorganic [45]. This discrepancy highlights the need to develop and compare commonly accepted sinking speed experiment methods in order to accurately constrain this key parameter on the biogeochemical models.
Table 2. Comparison of size, sinking velocities and excess densities observed with the inclusion of different type of ballast material incorporated with previous studies.
| Ballast material incorporated | Particle size range (mm) | Sinking speed range (m d-1) | Excess density range (mg cm-3) | Reference |
|---|---|---|---|---|
| Organic | 1-3.4 | 13-160 | 0.1-1.3 | This study |
| Opal | 1.2-3 | 23-191 | 0.14-0.92 | |
| Calcite | 1-2.9 | 45-283 | 0.3-2.3 | |
| Lithogenic | 1-3.7 | 41-322 | 0.26-4.44 | |
| Natural aggregates* | 0.3-0.6 | 160-280 | ns | [21] |
| Natural aggregates* | 1-20 | 20-200 | 0.01-10 | [7] |
| Calcite+saharian dusts (formed from natural seawater) | 1-6 | 100-600 | ns | [9] |
| Opal | 1-3 | 55-350 | 0.63-2.23 | [17] |
| Calcite | 1-2 | 25-63 | 3.36 | |
| Appendicularian fecal pellets* | 0.5-0.7 | 500-900 | 180-320 | |
| Copepod fecal peletts | 0.1 | 100-200 | 110-190 | |
| Opal | 1-5 | 40-200 | ns | [16] |
| Calcite | 1-4 | 100-325 | ns | |
| Opal+calcite | 1-4 | 50-200 | ns | |
| Calcite (several concentrations) | 0.9-4.5 | ns | 0.2-56 | [45] in [34] |
| Calcite - low CO2 | 2-4 | 850-1700 | 0.008-8 | [19] |
| Calcite - medium CO2 | 2-3 | 432-1000 | 0.008-9 | |
| Calcite - high CO2 | 2.5-5 | 170-600 | 0.008-10 | |
| E . huxleyi | [20] | |||
| Calcite | 0.7-2.4 | 86-1800 | 2.1-41 | |
| Organic | 0.8-11 | 950-2160 | 0.02-15 | |
| Opal+lithogenic | 0.08-0.6 | 200-400 | 2-100 | [44] |
| Opal+lithogenic | 0.1-0.8 | 300-800 | 2-200 | |
| Opal+lithogenic (various origin) | 0.1-0.6 | 200-1000 | 1-100 |
in situ formed aggregates
The ballast type affects the sinking speed of appendicularian houses. Opal has a small effect on particles sinking speed, increasing it by ~50% while calcite and lithogenic (Saharan dust) material significantly increase the particles’ sinking speed by ~100 and ~150%, respectively. These differences are related to the specific ballast density, 2.71 g cm-3 for calcite, 2.65 g cm-3 for quartz, and 2.1 g cm-3 for opal, although the silicifying and calcifying species used here may have lower densities because they include organic matter from the phytoplankton [12]. Additionally, the aggregates studied here are a combination of house material and particles trapped inside it, which have an organic and inorganic fraction. This explains the lower than expected aggregate densities observed here.
It has been suggested that calcite has a higher carrying capacity than opal, allowing more carbon to be carried to depth on calcite particles relative to opal [12]. Therefore, the larger increase of sinking speed caused by calcite ballast may explain the more efficient transfer of organic matter to the seafloor by calcite than opal. However, this has to be taken with caution, since the higher carrying capacity for calcite may simply reflects a stronger global correlation between calcite and organic carbon and as such is a statistical description rather than an explicit mechanism [46]. Recent sediment trap data suggest that other factors, such as remineralization rates and ecosystem function, are also key in controlling the organic carbon reaching the seafloor [47,48]. Interestingly, it has been suggested that high latitude diatom-dominated areas are characterized by low transfer efficiency in the water column [47-49], which have been attributed to poorly packaged aggregates that disintegrate easily or are more easily remineralized. Alternatively, it could also represent a faster utilization of sinking material by the food chain [50], notably by zooplankton that intensively feed on diatom sinking aggregates [51], while microbial processes may control the transfer efficiency in picophytoplankton dominated regions, leading to a better transfer of sinking material [50]. Our results gives one additional explanation by showing that opal have only a minor influence on aggregates sinking speed compared to calcite or lithogenic particles, which also partly explain the more efficient transfer of organic matter to the seafloor by calcite than opal.
Acknowledgments
We thank Cornelia Jaspers for providing the strain of coccolithophorids and Cécile Guieu for providing the Saharan dusts. We also thank the three anonymous reviewers for their improvement of the manuscript.
Funding Statement
Funding was provided by a Marie Curie Intra-European Fellowship 221696 award to F.L. and the French program Agence Nationale de la Recherche ANR-10-PDOC-005-01 "Ecogely" to F.L. The Danish Council for Independent Research provided further support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Buesseler KO, Lamborg CH, Boyd PW, Lam PJ, Trull TW et al. (2007) Revisiting carbon flux through the ocean’s twilight zone. Science 316: 567-570. doi:10.1126/science.1137959. PubMed: 17463282. [DOI] [PubMed] [Google Scholar]
- 2. Fowler SW, Knauer GA (1986) Role of large particles in the transport of elements and organic compounds through the ocean water column. Prog Oceanogr 16: 147-194. doi:10.1016/0079-6611(86)90032-7. [Google Scholar]
- 3. Volk T, Hoffert MI (1985) The Carbon Cycle and Atmospheric CO2: Natural Variations Archean to Present. In: Sundquist ET, Broecker WS. Geophysical Monograph. pp. 99-110. [Google Scholar]
- 4. Boyd PW, Trull TW (2007) Understanding the export of biogenic particles in oceanic waters: Is there consensus? Prog Oceanogr 72: 276-312. doi:10.1016/j.pocean.2006.10.007. [Google Scholar]
- 5. Burd AB, Hansell DA, Steinberg DK, Anderson TR, Aristegui J et al. (2010) Assessing the apparent imbalance between geochemical and biochemical indicators of meso- and bathypelagic biological activity: What the @$#! is wrong with present calculations of carbon budgets? Deep Sea Res II Topical Stud Oceanogr 57: 1557-1571. doi:10.1016/j.dsr2.2010.02.022. [Google Scholar]
- 6. Turner JT (2002) Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol 27: 57-102. doi:10.3354/ame027057. [Google Scholar]
- 7. Alldredge AL, Gotschalk C (1988) In situ settling behavior of marine snow. Limnol Oceanogr 33: 339-351. doi:10.4319/lo.1988.33.3.0339. [Google Scholar]
- 8. Guidi L, Jackson GA, Stemmann L, Miquel JC, Picheral M et al. (2008) Relationship between particle size distribution and flux in the mesopelagic zone. Deep Sea Res I Oceanogr Res Pap 55: 1364-1374. doi:10.1016/j.dsr.2008.05.014. [Google Scholar]
- 9. Iversen MH, Nowald N, Ploug H, Jackson GA, Fischer G (2010) High resolution profiles of vertical particulate organic matter export off Cape Blanc, Mauritania: Degradation processes and ballasting effects. Deep Sea Res I Oceanogr Res Pap 57: 771-784. doi:10.1016/j.dsr.2010.03.007. [Google Scholar]
- 10. Jouandet M-P, Trull TW, Guidi L, Picheral M, Ebersbach F et al. (2011) Optical imaging of mesopelagic particles indicates deep carbon flux beneath a natural iron-fertilized bloom in the Southern Ocean. Limnol Oceanogr 56: 1130-1140. doi:10.4319/lo.2011.56.3.1130. [Google Scholar]
- 11. Francois R, Honjo S, Krishfield R, Manganini S (2002) Factors controlling the flux of organic carbon to the bathypelagic zone of the ocean. Glob Biogeochem Cycles 16. [Google Scholar]
- 12. Klaas C, Archer DE (2002) Association of sinking organic matter with various types of mineral ballast in the deep sea: Implications for the rain ratio. Glob Biogeochem Cycles 16. [Google Scholar]
- 13. McDonnell AMP, Buesseler KO (2010) Variability in the average sinking velocities of marine particles. Limnol Oceanogr 55: 2085-2096. doi:10.4319/lo.2010.55.5.2085. [Google Scholar]
- 14. Armstrong RA, Lee C, Hedges JI, Honjo S, Wakeham SG (2002) A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep Sea Res II 49: 219-236. [Google Scholar]
- 15. Biermann A, Engel A (2010) Effect of CO2 on the properties and sinking velocity of aggregates of the coccolithophore Emiliania huxleyi . Biogeosciences 7: 1017-1029. doi:10.5194/bg-7-1017-2010. [Google Scholar]
- 16. Engel A, Szlosek J, Abramson L, Liu Z, Lee C (2009) Investigating the effect of ballasting by CaCO3 in Emiliania huxleyi: I. Formation, settling velocities and physical properties of aggregates. Deep Sea Res II Topical Stud Oceanogr 56: 1396-1407. doi:10.1016/j.dsr2.2008.11.027. [Google Scholar]
- 17. Iversen MH, Ploug H (2010) Ballast minerals and the sinking carbon flux in the ocean: carbon-specific respiration rates and sinking velocity of marine snow aggregates. Biogeosciences 7: 2613-2624. doi:10.5194/bg-7-2613-2010. [Google Scholar]
- 18. Riley JS, Sanders R, Marsay C, Le Moigne FAC, Achterberg EP et al. (2012) The relative contribution of fast and slow sinking particles to ocean carbon export. Glob Biogeochem Cycles 26. [Google Scholar]
- 19. Sanders R, Morris PJ, Poulton AJ, Stinchcombe MC, Charalampopoulou A et al. (2010) Does a ballast effect occur in the surface ocean? Geophys Res Lett 37. [Google Scholar]
- 20. Logan BE, Kilps JR (1995) Fractal dimensions of aggregates formed in different fluid mechanical environments. Water Res 29: 443-453. doi:10.1016/0043-1354(94)00186-B. [Google Scholar]
- 21. Flood PR, Deibel D (1998) The appendicularian house. In: Bone Q. The biology of pelagic tunicates. Oxford: Oxford University Press; pp. 105-124. [Google Scholar]
- 22. Lombard F, Selander E, Kiorboe T (2011) Active prey rejection in the filter-feeding appendicularian Oikopleura dioica . Limnol Oceanogr 56: 1504-1512. doi:10.4319/lo.2011.56.4.1504. [Google Scholar]
- 23. Sato R, Tanaka Y, Ishimaru T (2003) Species-specific house productivity of appendicularians. Mar Ecol Prog S 259: 163-172. doi:10.3354/meps259163. [Google Scholar]
- 24. Gorsky G, Chretiennot-Dinet MJ, Blanchot J, Palazzoli I (1999) Picoplankton and nanoplankton aggregation by appendicularians: Fecal pellet contents of Megalocercus huxleyi in the equatorial Pacific. J Geophys Res, C, Oceans 104: 3381-3390. [Google Scholar]
- 25. Passow U, Shipe RF, Murray A, Pak DK, Brzezinski MA et al. (2001) The origin of transparent exopolymer particles (TEP) and their role in the sedimentation of particulate matter. Contin Shelf Res 21: 327-346. doi:10.1016/S0278-4343(00)00101-1. [Google Scholar]
- 26. Hansen JLS, Kiørboe T, Alldredge AL (1996) Marine snow derived from abandoned larvacean houses: Sinking rates, particle content and mechanisms of aggregate formation. Mar Ecol Prog S 141: 205-215. doi:10.3354/meps141205. [Google Scholar]
- 27. Alldredge AL, Silver MW (1988) Characteristics, dynamics and significance of marine snow. Prog Oceanogr 20: 41-82. doi:10.1016/0079-6611(88)90053-5. [Google Scholar]
- 28. Alldredge AL (2005) The contribution of discarded appendicularian houses to the flux of particulate organic carbon from oceanic surface waters. In: Gorsky G, Youngbluth MJ, Deibel D. Response of marine ecosystems to global change: Ecological impact of appendicularians. Paris: GB. Scientific Publisher; pp. 309-326. [Google Scholar]
- 29. Robison BH, Reisenbichler KR, Sherlock RE (2005) Giant larvacean houses: Rapid carbon transport to the deep sea floor. Science 308: 1609-1611. doi:10.1126/science.1109104. PubMed: 15947183. [DOI] [PubMed] [Google Scholar]
- 30. Lombard F, Legendre L, Picheral M, Sciandra A, Gorsky G (2010) Prediction of ecological niches and carbon export by appendicularians using a new multispecies ecophysiological model. Mar Ecol Prog S 398: 109-125. doi:10.3354/meps08273. [Google Scholar]
- 31. De La Rocha CL, Passow U (2007) Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res I Oceanogr Res Pap 54: 639-658. [Google Scholar]
- 32. Bouquet JM, Spriet E, Troedsson C, Otterå H, Chourrout D et al. (2009) Culture optimization for the emergent zooplanktonic model organism Oikopleura dioica . J Plankton Res 31: 359-370. doi:10.1093/plankt/fbn132. PubMed: 19461862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lombard F, Renaud F, Sainsbury C, Sciandra A, Gorsky G (2009) Appendicularian ecophysiology I Food concentration dependent clearance rate, assimilation efficiency, growth and reproduction of Oikopleura dioica . J Mar Syst, 76: 151–161. doi:10.1016/j.jmarsys.2009.1001.1004. PubMed: 20628532.20628532 [Google Scholar]
- 34. Lombard F, Kiørboe T (2010) Marine snow originating from appendicularian houses: Age-dependent settling characteristics. Deep Sea Res I Oceanogr Res Pap 57: 1304-1313. doi:10.1016/j.dsr.2010.06.008. [Google Scholar]
- 35. Guieu C, Dulac F, Desboeufs K, Wagener T, Pulido-Villena E et al. (2010) Large clean mesocosms and simulated dust deposition: a new methodology to investigate responses of marine oligotrophic ecosystems to atmospheric inputs. Biogeosciences 7: 2765-2784. doi:10.5194/bg-7-2765-2010. [Google Scholar]
- 36. Lombard F, Sciandra A, Gorsky G (2005) Influence of body mass, food concentration, temperature and filtering activity on the oxygen uptake of the appendicularian Oikopleura dioica . Mar Ecol Prog S 301: 149-158. doi:10.3354/meps301149. [Google Scholar]
- 37. Fenaux R (1985) Rhythm of secretion of Oikopleurid’s houses. Bull Mar Sci 37: 498-503. [Google Scholar]
- 38. Tiselius P, Kuylenstierna M (1996) Growth and decline of a diatom spring bloom: Phytoplankton species composition, formation of marine snow and the role of heterotrophic dinoflagellates. J Plankton Res 18: 133-155. doi:10.1093/plankt/18.2.133. [Google Scholar]
- 39. Guerzoni S, Chester R, Dulac F, Herut B, Loye-Pilot MD et al. (1999) The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog Oceanogr 44: 147-190. doi:10.1016/S0079-6611(99)00024-5. [Google Scholar]
- 40. De La Rocha CL, Nowald N, Passow U (2008) Interactions between diatom aggregates, minerals, particulate organic carbon, and dissolved organic matter: Further implications for the ballast hypothesis. Glob Biogeochem Cycles 22. [Google Scholar]
- 41. Hamm CE (2002) Interactive aggregation and sedimentation of diatoms and clay-sized lithogenic material. Limnol Oceanogr 47: 1790-1795. doi:10.4319/lo.2002.47.6.1790. [Google Scholar]
- 42. Passow U, De la Rocha CL (2006) Accumulation of mineral ballast on organic aggregates. Glob Biogeochem Cycles 20. [Google Scholar]
- 43. Acuña JL, Keifer M (2000) Functional response of the appendicularian Oikopleura dioica . Limnol Oceanogr 45(3): 608-618. doi:10.4319/lo.2000.45.3.0608. [Google Scholar]
- 44. Ploug H, Iversen MH, Fischer G (2008) Ballast, sinking velocity, and apparent diffusivity within marine snow and zooplankton fecal pellets: Implications for substrate turnover by attached bacteria. Limnol Oceanogr 53: 1878-1886. doi:10.4319/lo.2008.53.5.1878. [Google Scholar]
- 45. Poulton AJ, Sanders R, Holligan PM, Stinchcombe MC, Adey TR et al. (2006) Phytoplankton mineralization in the tropical and subtropical Atlantic Ocean. Glob Biogeochem Cycles 20. [Google Scholar]
- 46. Wilson JD, Barker S, Ridgwell A (2012) Assessment of the spatial variability in particulate organic matter and mineral sinking fluxes in the ocean interior: Implications for the ballast hypothesis. Glob Biogeochem Cycles 26. [Google Scholar]
- 47. Henson SA, Sanders R, Madsen E (2012) Global patterns in efficiency of particulate organic carbon export and transfer to the deep ocean. Glob Biogeochem Cycles 26. [Google Scholar]
- 48. Le Moigne FAC, Sanders RJ, Villa-Alfageme M, Martin AP, Pabortsava K et al. (2012) On the proportion of ballast versus non-ballast associated carbon export in the surface ocean. Geophys Res Lett 39. [Google Scholar]
- 49. Lam PJ, Doney SC, Bishop JKB (2011) The dynamic ocean biological pump: Insights from a global compilation of particulate organic carbon, CaCO3, and opal concentration profiles from the mesopelagic. Glob Biogeochem Cycles 25. [Google Scholar]
- 50. Guidi L, Stemmann L, Jackson GA, Ibanez F, Claustre H et al. (2009) Effects of phytoplankton community on production, size and export of large aggregates: A world-ocean analysis. Limnol Oceanogr 54: 1951-1963. doi:10.4319/lo.2009.54.6.1951. [Google Scholar]
- 51. Kiørboe T (2000) Colonization of marine snow aggregates by invertebrate zooplankton: Abundance, scaling, and possible role. Limnol Oceanogr 45: 479-484. doi:10.4319/lo.2000.45.2.0479. [Google Scholar]