Figure 1. The roles of central and peripheral tolerance in the development and protection against T cell mediated autoimmune myocarditis.
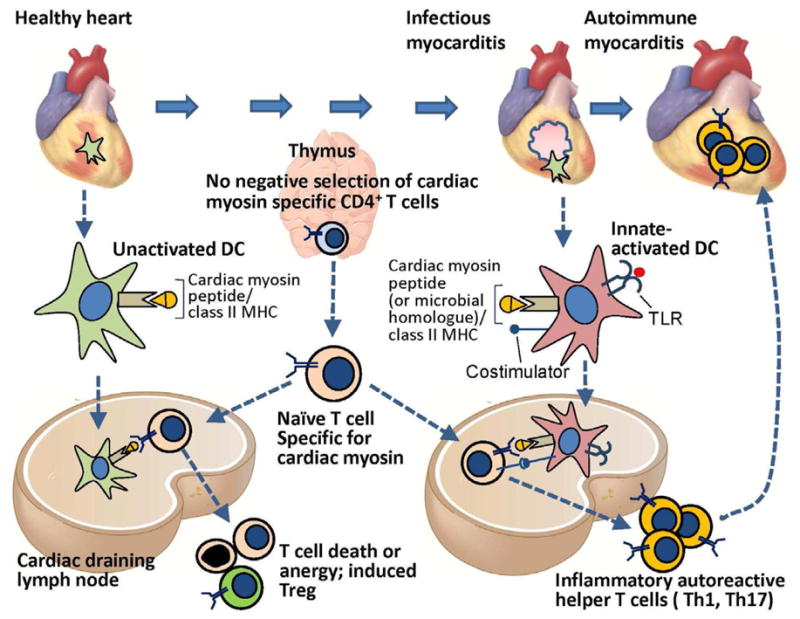
Central T cell tolerance to many peripheral tissue-specific protein antigens occurs by AIRE-dependent expression of these proteins in thymic epithelial cells (TMEC) and presentation of peptides derived from the proteins to developing antigen-specific T cells, leading to deletion of the T cells. Cardiac myosin (α-myosin heavy chain) is not expressed by TMEC, and therefore α-myosin heavy chain-specific T cells emerge from the thymus. These myocardial-antigen specific naïve T cells and are found in the blood of mice and humans, and during their recirculation, migrate through the cardiac draining lymph node (CDLN). Dendritic cells that display α myosin peptides in complex with class II MHC are present in the healthy heart, and these resting tolerogenic DCs also traffic to the CDLN, where they present the α myosin peptides to the naïve α-myosin heavy chain-specific CD4+ T cells, leading to deletion, anergy or Treg induction. If the heart is infected (e.g. by coxsackievieus B virus, T Cruzi) or is damaged by ischemic infarction, myocardial DCs are activated by TLR ligands, become capable of productively activating cardiac myosin-specific naïve T cells in the CDLN, leading to differentiation of inflammatory effector CD4+ T cells that migrate back to the heart and cause autoimmune myocarditis. It is possible that T cells specific for microbial peptides that are homologous to myocardial antigens are also involved in autoimmune myocarditis. The susceptibility to developing T cell mediated myocarditis depends on inheritance of certain alleles of MHC genes as well as other autoimmune susceptibility genes.
