Figure 2.
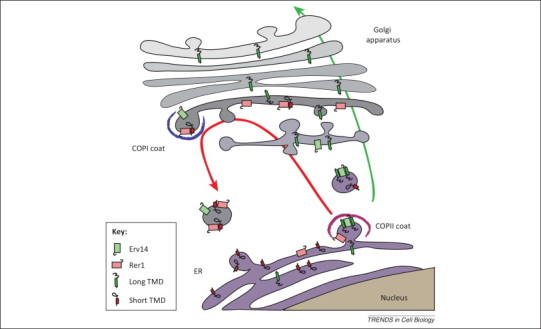
TMD recognition in the early secretory pathway. To be expressed at the cell surface, membrane proteins must be transported along the secretory pathway from the endoplasmic reticulum (ER) to the Golgi apparatus and then to the plasma membrane (green arrow). In the early steps of the secretory pathway, transmembrane domains (TMDs) are recognized at least at two distinct sites. During the formation of COPII-coated vesicles, ER proteins with long TMDs are concentrated in budding vesicles due to their interaction with Erv14 and are thus efficiently transported to the cis-Golgi apparatus and beyond. By contrast, proteins with short TMDs are bound by Rer1 in the cis-Golgi and concentrated in retrograde COPI-coated vesicles destined for the ER (red arrow). These two systems, probably in concert with partitioning into different lipid domains, ensure efficient transport of proteins with long TMDs along the secretory pathway and localization of proteins with short TMDs to the ER. This model assumes that both Erv14 and Rer1 bind their targets in a regulated manner, to capture them in one compartment (e.g., the ER for Erv14) and release them in another (e.g., the Golgi for Erv14) before returning to their original location, but the mechanisms involved are unknown. This simplified scheme also does not depict other putative sorting receptors involved in the export of distinct subsets of membrane proteins from the ER.
