Abstract
Objective:
This study aims to phytochemical and antimicrobial study of Euphorbia hirta Euphorbiaceae).
Materials and Methods:
Antimicrobial activity of flavonoids (free and bound) of Euphorbia hirta L. was determined by disc diffusion assay against four bacteria (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, and Staphylococcus aureus) and four fungi (Aspergillus flavus, Aspergillus niger, Trichophyton mentagrophytes, and Candida albicans). Minimum inhibitory concentration (MIC) of the extract was evaluated through micro broth dilution method, while minimum bactericidal/fungicidal concentration was determined by subculturing the relevant samples. Total activity (TA) of extracts against each sensitive pathogen was also evaluated.
Results:
Out of fungi; A. flavus, A. niger, and T. mentagrophytes were found to be resistant, against which none of the tested extracts showed activity. Bound flavonoids extract of root showed best activity against C. albicans (inhibition zone (IZ) 27.66, MIC 0.039, minimum fungicidal concentration (MFC) 0.039). TA of free flavonoid extract of root was found to be the same for P. mirabilis and S. aureus (192.30 ml/g). Two flavonoids quercetin and kaempferol were identified in the bound flavonoids of stem extract which showed activity against all the microorganisms.
Conclusion:
Results of the present investigation indicate that E. hirta has good antimicrobial activity with low range of MIC, hence can be exploited for future plant-based antimicrobial drugs.
Keywords: Euphorbia hirta, flavonoid, kaempferol, minimum inhibitory concentration, quercetin, total activity
INTRODUCTION
Infectious diseases are the leading cause of death worldwide. Antibiotic resistance has become a global concern.[1] The clinical efficacy of many existing antibiotics is being threatened by the emergence of multidrug resistant pathogens.[2] Many infectious diseases have been known to be treated with herbal remedies throughout the history of mankind. There is a continuous and urgent need to discover new antimicrobial compounds with diverse chemical structures and novel mechanisms of action for new and reemerging infectious diseases.[3] Medicinal plants are gifts of nature to cure limitless number of diseases of human beings.[4] The abundance of plants on the earth's surface has led to an increasing interest in the investigation of different extracts obtained from traditional medicinal plants, as potential source of new antimicrobial agents.[5] Hence, researchers are increasingly turning their attention to folk medicine, looking for new leads to develop better drug against microbial infections.[6] Increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic microbial infectious agents has led to screening of several medicinal plants for their potential antimicrobial activity.[7,8] In recent years, secondary plant metabolites (phytochemicals), previously with unknown pharmacological activities, have been extensively investigated as a source of medicinal agents.[9]
Euphorbia hirta belonging to family Euphorbiaceae is a medicinal, rhizomatous herb distributed in southern Western Ghats of India and northern east coast of Tamil Nadu.[10] In east and west Africa, extracts of the plant are used in treatment of asthma and respiratory tract inflammations.[11] It is also used for coughs, chronic bronchitis, and other pulmonary disorders in Malagasy.[12] The plant is also widely used in Angola against diarrhea and dysentery, especially amebic dysentery. In Nigeria, extracts or exudates of the plant are used as ear drops and in the treatment of boils, sore, and promoting wound healing.[13]
In the current investigation E. hirta showed its antimicrobial potential against test pathogens, which are being involved in a number of human diseases. E. hirta has previously been studied for antibacterial and antifungal activities, but still the literature available is meager. Antibacterial activity and toxicological potentials of crude ethanolic extracts of E. hirta against S. aureus, E. coli, P. aeruginosa, Salmonella typhi, and Bacillus subtilis.[14] Aqueous, methanol, hexane, and amoxicillin extracts of E. hirta have been found to be active against E. coli, P. mirabilis, Shigella dysentriae, Salmonella typhi, and Klebsiella pneumoniae.[15] Methanolic extract of leaf inhibited the growth of S. aureus, E. coli, and B. subtilis.[16]
Screening of the plant under investigation (E. hirta) so far has not been worked out for flavonoids. Mostly the crude extracts have been screened, that too without Minimum inhibitory concentration (MIC), minimum bactericidal/fungicidal concentration (MBC/MFC), and total activity (TA) determination. Such studies could only indicate their antimicrobial potential, but are not helpful in establishing them as an antibiotic.
The aim of the study was to investigate the antimicrobial activity of extracts of E. hirta in order to explore possibility for new antimicrobial substances against some human pathogens. The present investigation evaluates the antibacterial and antifungal effects of free and bound flavonoids of E. hirta (root, stem, leaf, and fruits). The study was carried out along with standard drugs (terbinafine and itraconazol for fungus and streptomycin for bacteria).
MATERIALS AND METHODS
Plant material
Different parts (root, stem, leaf, and fruits) of E. hirta were collected from Jaipur (India), and the specimen of the plant was identified at the Department of Botany, University of Rajasthan. The sample specimen with No. RUBL20666 was submitted in the ‘Herbarium’ of Department pf Botany, University of Rajasthan.
Extraction procedure
Plant parts were separately shade dried and finely powdered using a mixer. Free and bound flavonoids from root, stem leaf, and fruits of E. hirta was extracted following the well-established method.[17] Hundred grams of each finely powered sample was Soxhlet extracted with 80% hot methanol (500 ml) on a water bath for 24 h and filtered. Each filtrate was reextracted successively with petroleum ether (fraction I), ethyl ether (fraction II), and ethyl acetate (fraction III) using separating funnel. Petroleum ether fractions were discarded as being rich in fatty substances, whereas ethyl ether and ethyl acetate fractions were analyzed for free and bound flavonoids, respectively. Ethyl acetate fraction of each of the samples was hydrolyzed by refluxing with 7% H2SO4 for 2 h (for removal of bound sugars from the flavonoids) and filtered. The filtrate was extracted in ethyl acetate and washed with distilled water to neutrality. Ethyl ether (free flavonoid) and ethyl acetate fractions (bound flavonoids) thus obtained were dried in vaccuo and weighed. The extracts were stored at 4°C and were resuspended in their respective solvents to get 10 mg/ml for antimicrobial assay.
Identification
Selected extract (bound flavonoid of stem) which showed activity against all microorganisms tested was dissolved in ethyl acetate and applied on silica gel coated (0.2-0.3 mm) and activated glass plates (20 × 20 cm) in an oven at 100°C for 30 min along with the standard reference compound of apigenin and quercetin 1 cm above the edge of the plates. These plates were developed in an organic solvent mixture of benzene, acetic acid, and water (125:72:3), air dried and visualized under ultraviolet (UV) light. Three spots (retention factor (Rf) 0.86, 0.78, 0.10) were observed which were further confirmed by spraying the plates with 5% ethanolic ferric chloride solution [Table 1 and Figure 1]. A few other solvent system (n-butanol:acetic acid: water, 4:1:5; n-butanol: Water 1:1; n-butanol:acetic acid:water, 6:1:2) were used, but in the present investigation the solvent system of benzene, acetic acid, and water (125:72:3) gave excellent results.[18] Rf value 0.86, 0.78 obtained indicate the presence of kaempferol and quercetin in the bound flavonoids of stem extract subjected to thin layer chromatography (TLC).
Table 1.
Rf values of spots of flavonoid extract obtained in TLC (solvent system benzene:acetic acid: Water 125:72:3)

Figure 1.

Thin layer chromatography. E: Extract; K: Kaempferol; Q: Quercetin
Preparative TLC of the bound flavonoids from stem of E. hirta was carried out on silica gel coated and activated (0.4-0.5 mm thick) glass plates in the selected solvent (benzene, acetic acid, and water). Spot of Rf value 0.86 and 0.78 were marked on each plate and were collected and eluted with ethyl acetate. Elutes were pooled, completely dried, and rechromatographed to test the purity of the isolated compound.
The isolated compounds were crystallized, weighed, and subjected to melting point (m.p.) and infrared spectral studies on Perkins Elmer model 555 spectrophotometer in KBr pellets. Quercetin (Rf 0.78; UV fluorescent-dull yellow, ammonia-yellow brown, FeCl3-blue-grey, m.p. 309-311°C) and Kaempferol (Rf 0.86; UV fluorescent - bright yellowish blue, ammonia - deep yellow, FeCl3 - brown; m.p. 270-273°C) were identified in bound flavonoids stem of E. hirta [Figure 2].
Figure 2.
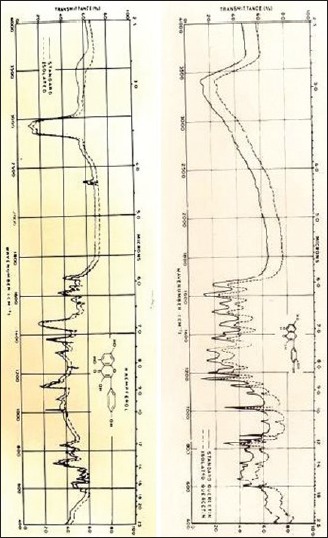
Infrared of quercetin and kaempferol
Test pathogens
Eight pathogenic microorganisms in total, including four bacteria, viz., E. coli (MTCC no. 46), P. aeruginosa (MTCC 1934), P. mirabilis (MTCC 3310), and S. aureus (MTCC 3160); and four fungal strains, viz., C. albicans (MTCC 183), A flavus (MTCC 277), A. niger (MTCC 282), and Trichophyton mentagrophytes (MTCC no. 7687) were procured from IMTECH (Chandigarh, Punjab, India). Bacterial strains were grown and maintained on Mueller-Hinton agar (MHA) medium, while fungi were maintained on Sabouraud dextrose agar medium (SDA).
Screening for antimicrobial activity
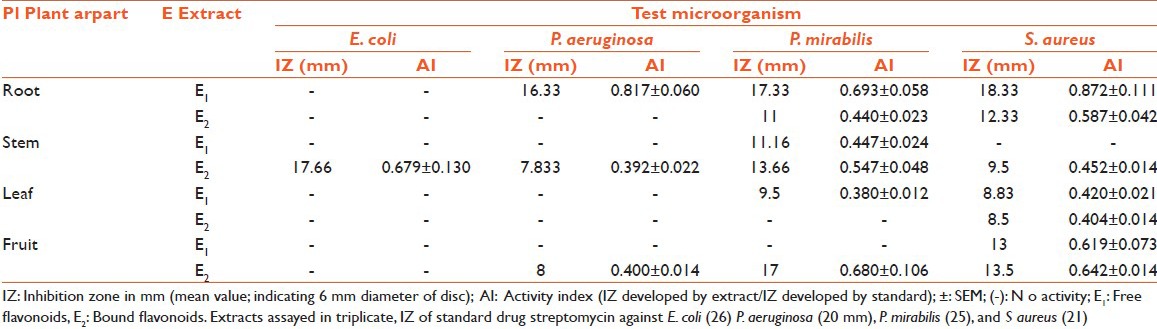
Disc diffusion assay was performed for antimicrobial screening.[19] MHA and SDA base plates were seeded with the bacterial and fungal inoculum, respectively with inoculum size 1 × 108 CFU/ml for bacteria and 1 × 107 cell/ml for yeast. Sterile filters paper discs (Whatman no. 1, 6 mm in diameter) were impregnated with 100 μl of each of the extract (10 mg/ml) to give a final concentration of 1 mg/disc and left to dry in vaccuo so as to remove residual solvent, which might interfere with the determination. Extract discs were then placed on the seeded agar plates. Each extract was tested in triplicate with streptomycin (1 mg/disc) and candid-V6 (1 mg/ml) as standard for bacteria and fungi, respectively. The plates were kept at 4°C for 1 h for diffusion of extract, thereafter were incubated at 37°C for bacteria (24 h) and 27°C for fungi (48 h). Antibacterial and antifungal activity was expressed in terms of activity index (AI). AI for each extract was calculated [Table 2].
Table 2.
Antibacterial activity of flavonoids of Euphorbia hirta by disc diffusion assay
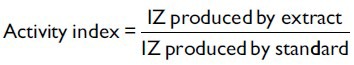
where IZ = inhibition zone.
Determination of MIC and MBC/MFC
MIC was determined for the plant extract showing antimicrobial activity against test pathogens. Broth microdilution method was followed for determination of MIC values.[20] Plant extracts were resuspended in acetone (which has no activity against test pathogens) to make 10 mg/ml final concentration and then was two-fold serially diluted. Each dilution was added to broth media of 96-wells of microtiter plates. Thereafter 100 μl inoculum (for bacteria 1 × 108 CFU/ml and for yeast and fungi 1 × 107 CFU/ml) was added to each well. Bacterial and fungal suspensions were used as negative control, while broth containing standard drug was used as positive control. The microtiter plates were incubated at 37°C for 24 h for bacteria and 28°C for 48 h for yeast. Each extract was assayed in duplicate and each time two sets of microplates were prepared, one was kept for incubation while another set was kept at 4°C for comparing the turbidity in the wells of microplate. The MIC values were taken as the lowest concentration of the extracts in the well of the microtiter plate that showed no turbidity after incubation. The turbidity of the wells in the microtiter plate was interpreted as visible growth of microorganisms. The MBC/MFC was determined by subculturing 50 μl from each well. Least concentration of extract showing no visible growth on subculturing was taken as MBC/MFC.
TA determination
TA is the volume at which the test extract can be diluted with the ability to kill the microorganisms. It is calculated by dividing the amount of extract from 1 g plant material by the MIC of the same extract or compound isolated and is expressed in ml/g.[21]
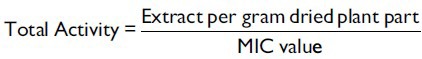
RESULTS AND DISCUSSION
Antimicrobial potency of flavonoids (free and bound) of E. hirta was assessed by IZ and AI [Tables 2 and 3]. Free and bound flavonoid extract exhibited good inhibitory activity against most of the pathogens. In the present study, total eight extracts of different parts of plants were tested for their bioactivity. All eight extracts showed significant antimicrobial activity against test microbes. In all test microbes most susceptible organism in the investigation was C. albicans against which all the plant extracts showed IZ.
Table 3.
Antifungal activity of flavonoids of Euphorbia hirta by disc diffusion assay
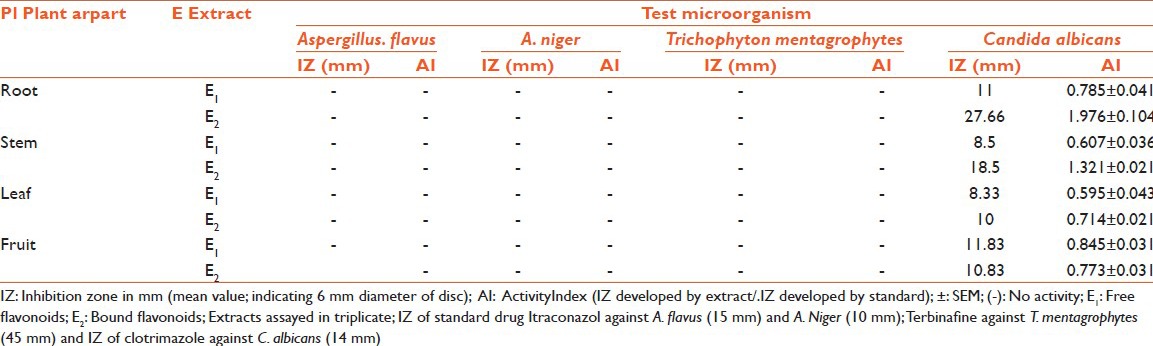
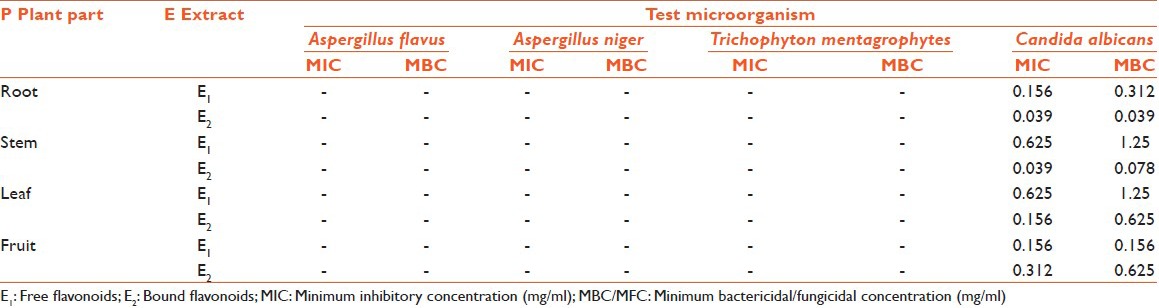
In some cases, even more than the standard and the best activity were observed for bound flavonoids of root with IZ 27.66 mm, AI 1.976 ± 0.104, MIC 0.039 mg/ml, and MFC 0.039. Out of the four bacteria tested, S. aureus was found to be the most sensitive whereas E. coli was found to be the most resistant microbe against which only one extract (bound flavonoid extract of stem) showed activity (IZ 17.66 mm, AI 0.679 ± 0.130, MIC 0.078 mg/ml, MFC 0.078). Best antibacterial activity was observed against P. aeruginosa (IZ 16.33 mm, AI 0.817 ± 0.060, MIC 0.156 mg/ml, MFC 0.156), P. mirabilis (IZ 17.33 mm, AI 0.693 ± 0.058, MIC 0.039 mg/ml, MFC 0.078), and S. aureus (IZ 18.33 mm, AI 0.872 ± 0.111, MIC 0.039, MFC 0.078) for free flavonoids of root; whereas, bound flavonoids of fruits showed best activity against P. mirabilis (IZ 17 mm, AI 0.680 ± 0.106, MIC 0.039 mg/ml, MFC 0.039). Free flavonoids of stem showed bioactivity against P. mirabilis (IZ 11.16 mm, AI 0.447 ± 0.024, MIC 0.156 mg/ml, MFC 0.312); whereas, bound flavonoids showed activity against C. albicans (IZ 18.5 mm, AI 1.321 ± 0.021, MIC 0.039 mg/ml, MFC 0.078). The growth of fungi (A. flavus, A. niger, and T. mentagrophytes) was not influenced by any of the test extracts. MIC and MBC/MFC values [Tables 4 and 5] were evaluated for those plant extracts, which showed activity. The range of MIC and MBC/MFC of extracts recorded was 0.039-0.625 mg/ml and 0.039-1.25 mg/ml, respectively. In the present investigation lowest MIC value (0.039 mg/ml) was recorded against P. mirabilis, S. aureus, and C. albicans; whereas, it was 0.078 mg/ml against E. coli and 0.156 mg/ml against P. aeruginosa.
Table 4.
MIC and MBC/MFC values of Euphorbia hirta against bacterial pathogens
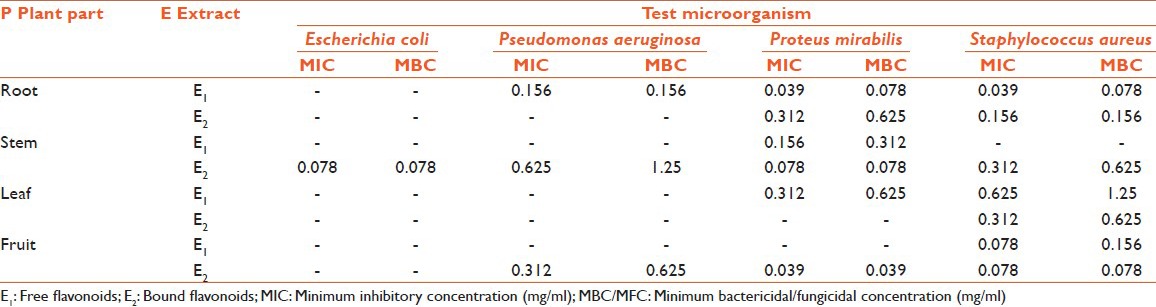
Table 5.
MIC and MBC/MFC values of Euphorbia hirta against fungal pathogens
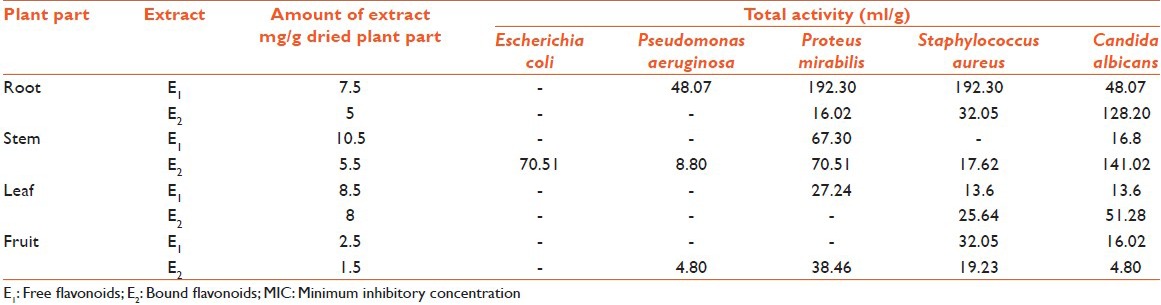
TA and quantity of extracts from plant parts was calculated and recorded [Table 6]. TA indicates the volume at which extracts can be diluted without losing the ability to kill microorganisms. Full TA calculated against E. coli, P. aeruginosa, P. mirabilis, S. aureus, and C. albicans, were 70.51, 48.07, 192.30, 192.30, and 141.02 ml, respectively. Most of the extracts showed high values of TA against P. mirabilis, S. aureus, and C. albicans; which proves potential of the extracts to inhibit growth of the test microorganisms, even at low concentration.
Table 6.
Quantity and total activity of free and bound flavonoids of Euphorbia hirta
The potential for developing antimicrobials from higher plants appears rewarding as it will lead to the development of phytomedicine to act against microbes. Plant-based antimicrobials have enormous therapeutic potential as they can serve the purpose of synthetic antimicrobials. Continued further exploration of plant-derived antimicrobials is need of the day.
Results of the present study reveals that all the eight tested plant extracts inhibit the growth of selected bacteria and fungi; indicating broad spectrum bioactive nature of selected plant. Most of the extracts of E. hirta were found to be potent inhibitor of tested organisms except E. coli, against which only one extract of the plant showed activity. Excellent activity was shown by free and bound flavonoids of roots of E. hirta as low values of MIC and MBC/MFC were observed.
Higher values of MBC/MFC than that of the MIC indicated the bacteriostatic/fungistatic nature of the extracts, which were observed for rest of the active extracts. Same values of MIC and MBC/MFC was observed for free flavonoids of root (0.156) against P. aeruginosa which shows their bactericidal nature, bound flavonoids of stem (0.078) against E. coli and P. mirabilis, and bound flavonoids of fruits (0.078) against S. aureus. MIC and MBC/MFC values were observed to be good enough for eight extracts of E. hirta which is a desirable character as far as the exploitation of compound for future plant based-drug is concerned.
Gram positive bacteria (S. aureus) was the second most susceptible organism after fungi C. albicans, which supported the finding that plant extracts are usually more active against gram positive bacteria than gram negative.[22,23] Susceptibility difference between gram positive and gram negative bacteria might be due to differences in cell wall structures.
Present investigation proves the plant under study to possess broad spectrum antimicrobial activity.
ACKNOWLEDGEMENT
Authors are thankful to the Head of Department of Botany, University of Rajasthan for providing all necessary facilities for present work. Financial assistance provided by UGC is gratefully acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Westh H, Zinn CS, Rosdahl VT. An international multicenter study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microb Drug Resist. 2004;10:169–76. doi: 10.1089/1076629041310019. [DOI] [PubMed] [Google Scholar]
- 2.Bandow JE, Brotz H, Leichert LI, Labischinski H, Hecker M. Proteomic approach to understanding antibiotic action. Antimicrob Agents Chemother. 2003;47:948–55. doi: 10.1128/AAC.47.3.948-955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beegum BN, Devi GT. Antibacterial activity of selected Seaweads from Kovalan south West coast of India. Asian Jr. of Microbiol. Biotech Env Sc. 2003;5:319–22. [Google Scholar]
- 4.Bonjar G, Farrokhi PR. Antibacillus activity of some plants used in traditional medicine of Iran. Niger J Nat Prod Med. 2004;8:34–9. [Google Scholar]
- 5.Rojas R, Bustamante B, Bauer J, Fernández I, Albán J, Lock O. Antimicrobial activity of selected Peruvian medicinal plants. J Ethnopharmacol. 2003;88:199–204. doi: 10.1016/s0378-8741(03)00212-5. [DOI] [PubMed] [Google Scholar]
- 6.Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) Lebensm-Wiss-U-Technol. 2004;37:263–8. [Google Scholar]
- 7.Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae) Pharmacol Res. 1996;33:127–34. doi: 10.1006/phrs.1996.0019. [DOI] [PubMed] [Google Scholar]
- 8.Iwu MW, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on New Crops and New Uses. Alexandrai: ASHS Press; 1999. pp. 457–62. [Google Scholar]
- 9.Krishnaraju AV, Rao TV, Sundararaju D, Vanisree M, Tsay HS, Subbaraju GV. Assessment of bioactivity of Indian medicinal plants using Brine Shrimp (Artemia salina) lethality assay. Int J Appl Sci Eng. 2005;2:125–34. [Google Scholar]
- 10.Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K. Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) Parasitol Res. 2008;102:867–73. doi: 10.1007/s00436-007-0839-6. [DOI] [PubMed] [Google Scholar]
- 11.Kokwaro JO. 2nd ed. Nairobi, Kenya: East African Literature Bureau; 1993. Medicinal Plants in East Africa. [Google Scholar]
- 12.Wong-Ting-Fook WT. Dakar: ENDA publication No. 10; 1980. The medicinal plants of Mauritius. [Google Scholar]
- 13.Igoli JO, Ogaji OG, Tor-Anyiin TA, Igoli NP. Traditional medicine practice amongst the igede people of Nigeria. Part II. Afr J Trad CAM. 2005;2:134–52. [Google Scholar]
- 14.Ogueke CC, Ogbulei JN, Okoli IC, Anyanwu N. Antimicrobial activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta. J Am Sci. 2007;3:11–6. [Google Scholar]
- 15.Abubakar ME. Antibacterial acitivity of crude extracts of Euphorbia hirta against some bacteria associated with enteric infections. J Med Plant Res. 2009;3:498–505. [Google Scholar]
- 16.Upadhyay B, Singh KP, Kumar A. Pharmacognostical and antibacterial studies of leaf extracts of Euphorbia hirta L. J Phytol. 2010;2:55–60. [Google Scholar]
- 17.Subramanian SS, Nagarjan S. Flavonoids of the seeds of Crotolaria retusa and Ctotolaria striata. Curr Sci. 1969;38:65. [Google Scholar]
- 18.Wong E, Francis CM. Flavonoids in genotypes of Trifolium subterraneum. I. The normal flavonoids pattern of the geraldton variety. Phytochem. 1968;7:2123–19. [Google Scholar]
- 19.Andrews JM. BSAC Working Party On Susceptibility Testing ft. BSAC standardized disc susceptibility testing method. J Antimicrob Chemother. 2001;4:43–57. doi: 10.1093/jac/48.suppl_1.43. [DOI] [PubMed] [Google Scholar]
- 20.Barsi DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J Pharmacol. 2005;37:26–9. [Google Scholar]
- 21.Eloff JN. Quantifying the bioactivity of the plant extracts during screening and bioassay guided fractionation. Phytomedicine. 2004;11:370–1. doi: 10.1078/0944711041495218. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Opake AR, Geheeb-Keller M, Hutchings AD, Terblanche SE, Jager AK. Preliminary screening of some traditional zulu medicinal plants for anti-inflammatory and anti-microbial activities. J Ethanopharmacol. 1999;68:267–74. doi: 10.1016/s0378-8741(99)00130-0. [DOI] [PubMed] [Google Scholar]
- 23.Polombo EA, Semple SJ. Antibacterial activity of traditional Australian medicinal plants. J Ethnopharmacol. 2001;77:151–7. doi: 10.1016/s0378-8741(01)00290-2. [DOI] [PubMed] [Google Scholar]


