Abstract
Sex cord stromal tumor with annular tubules (SCTAT) is a distinctive, rare subtype of sex cord stromal tumor of the ovary, predominant component of which has morphological features intermediate between that of granulosa cell and sertoli cell. The majority of ovarian SCTAT are benign. So far, malignant behavior in SCTAT has been reported only in sporadic cases. We have presented a case of malignant SCTAT in a 35-year-old lady with no associated Peutz-Jegher (P-J) syndrome.
Keywords: Annular tubules, Peutz-Jegher syndrome, sex cord stromal tumor
INTRODUCTION
Robert Scully in 1970 first described 13 cases of this unusual variant of sex cord stromal tumor.[1] Young et al., reviewed a large series (74 cases) including those associated with P-J syndrome.[2] In one third of the cases, hyperestrinism and P-J syndrome association was seen.[3] SCTATs with P-J syndrome are small, <3 cm, benign, bilateral, and multicentric in young women, whereas, in the absence of this syndrome, it is seen as huge, unilateral tumor, and approximately one-fifth (15-20%) tend to be malignant,[1,2,3,4,5] comprising only 1.2% of all cases of ovarian cancer.[6]
CASE REPORT
A 32-year-old multiparous lady was admitted for abdominal pain. On pelvic examination, a mass separate from the uterus was felt in the fornices. Ultrasonography showed a well-defined, thickwalled, lobulated, cystic ovarian mass with irregular septae measuring 17 × 16 × 6 cm. As the findings were suggestive of malignant ovarian tumor, she underwent panhysterectomy. Uterus, cervix, and left adnexa were unremarkable. The right ovary was cystic and measured 21 × 18 × 5 cm with a smooth external surface. The cut surface was multiloculated, filled with hemorrhagical fluid with focal solid areas [Figure 1a]. Microscopically, the cervix showed diffuse laminar endocervical hyperplasia. No adenoma malignum or mucinous metaplasia of endometrial or tubal epithelium was seen. Right ovary was replaced by a solid, cystic tumor composed of round to oval granulosa cells with vesicular nuclei and an occasional nuclear groove. Cytoplasm was moderate to scant and eosinophilic. Cells were arranged in sheets, nests, micro follicular, macro follicular, and insular pattern [Figure 1b]. Simple and complex tubules encircling nodules of hyalinised PAS-positive basement membrane-like material were seen [Figures 1c and d]. Nuclei showed antipodal arrangement with focal sertoliform pattern [Figure 1e]. Luteinisation was evident in areas. The adjacent ovarian stroma showed invasion by the tumor and lymphatic emboli [Figure 1f]. A diagnosis of malignant SCTAT was rendered after confirmation by immunohistochemistry (IHC) for inhibin and calretinin.
Figure 1.
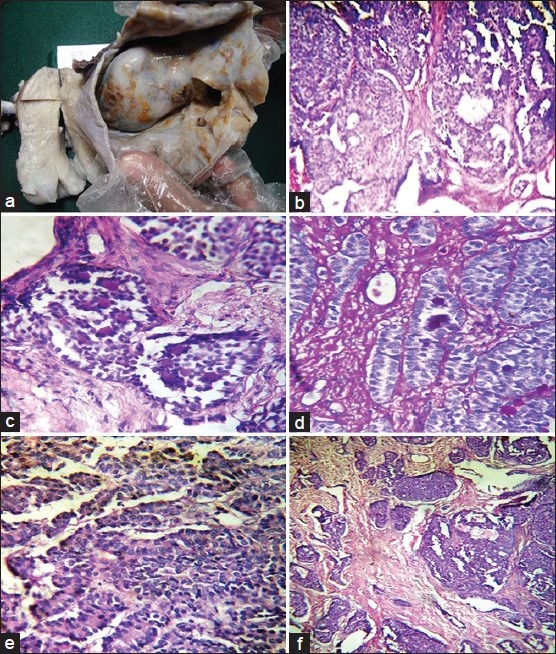
(a) Gross photograph showing solid cystic ovarian tumor. (b) Microscopic examination showing sheets and nests of cells arranged in microfollicular, macrofollicular, and insular pattern (H and E, ×100). (c and d) High-power view showing simple and complex tubules encircling nodules of hyalinised PAS-positive basement membrane-like material (H and E, ×400). (e) Sertoliform pattern with tubules lined by cells showing antipodal arrangement of nuclei (H and E, ×400). (f) Invasion into paraovarian tissue with numerous lymphatic emboli (H and E, ×100)
DISCUSSION
SCTAT was documented to be oestrogen- progesterone-secreting tumor based on the observation of glandular atrophy, decidual change of endometrial stroma, and assays of steroid hormone.[3] Age at presentation was 20-30 years with a mean age of 20.6 years.[3] Symptoms suggestive of hyperestrinism such as meno-metrrhagoia, followed by persistent amenorrhea, postmenopausal bleed, sexual precocity, and/or pelvic mass were presenting features.[1,2,3,4,5,6,7] Association with P-J syndrome and adenoma malignum of cervix is known.[1,2,3,4,5,6,7] When patients with ovarian tumor present with amenorrhea preceded by meno-metorrhagia, endometrial sampling should be done to look for glandular atrophy and decidual change to rule out the possibility of SCTAT. Our case presented at the age of 35 years with abdominal pain. She had no associated P-J syndrome or adenoma malignum of cervix, although diffuse laminar endocervical hyperplasia was noted. Setoli-Leydig cell tumor presents with similar symptoms and may morphologically simulate SCTAT. Steroid assay including estradiol, progesterone, and testosterone are helpful in differentiating the two. In Setoli-Leydig cell tumor, all three-estradiol, progesterone, and testosterone levels-are elevated. If both estradiol and progesterone are elevated with normal testosterone levels, the diagnosis of SCTAT is ascertained. Morphologically, these tumors are solid, tan to yellow colored with few tiny cysts and focal calcification. Microscopically, circumscribed epithelial nests composed of ring-shaped tubules are seen with antipodal nuclear arrangement encircling hyaline globules, which is continuous with the basement membrane. The rings have two patterns: single tubule with a central rounded hyaline mass and complex communicating tubules encircling multiple hyaline masses. Our case showed similar morphological features. Previous reports of both benign and malignant SCTAT, including both sporadic and P-J syndrome-associated tumors, have described additional histological patterns such as sertoliform tubules, endometrioid areas, and foci of granulosa cell-like differentiation. On the basis of such findings, some authors have suggested that SCTAT represent variants of either Sertoli cell or granulosa cell tumors, whereas others regard SCTAT as a distinctive neoplasm with features intermediate between those of Sertoli cell and granulosa cell tumors.[7] Mullerian-inhibiting substance is a glycoprotein hormone produced by fetal sertoli cells. Sex cord stromal tumors secrete a large amount of this hormone. The degree of the elevation correlates well with the tumor burden.[1,2,3,4] Gustafson et al., have reviewed over 5,60,000 ovarian biopsies obtained over a period of 40 years and stated that the measurement of this substance may help detect persistent or recurrent disease.[4] Malignant SCTAT seems to spread mainly via the lymphatics with typical sites of tumor metastasis being the pelvic, para-aortic, and supraclavicular lymph nodes. Other sites of tumor recurrence and metastasis include the retroperitoneum, peritoneum, liver, kidney, and lung. Unilateral salpingo-oopherectomy together with ipsilateral pelvic and para-aortic lymphadenectomy is suggested as an effective treatment for SCTAT. Radiotherapy is reserved for local recurrence and distant metastasis.[3,4,5,6,7] Our case has tolerated the radiotherapy and is well after 1 year of follow-up.
ACKNOWLEDGMENT
Momin Altaf A, Additional Superintendent Police, Turchi Police Training School, Tasgaon. Dist: Sangli, Maharashtra. India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Scully RE. Sex cord stromal tumor with annular tubules a distinctive ovarian tumour of the Peutz-Jegher syndrome. Cancer. 1970;25:1107–21. doi: 10.1002/1097-0142(197005)25:5<1107::aid-cncr2820250516>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Young RH, Welch WR, Dickerson GR, Scully RE. Ovarian sex cord tumor with annular tubules: Review of 74 cases Including 27 with P-J syndrome and four with adenoma malignum of cervix. Cancer. 1982;50:1384–402. doi: 10.1002/1097-0142(19821001)50:7<1384::aid-cncr2820500726>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Shen K, Wu PC, Lang JH, Huang RL, Tang MT, Lian LJ. Ovarian sex cord tumor with annular tubules: A report of six cases. Gynecol Oncol. 1993;48:180–4. doi: 10.1006/gyno.1993.1030. [DOI] [PubMed] [Google Scholar]
- 4.Gustafson ML, Lee MM, Scully RE, Moncure AC, Hirakawa T, Goodman A, et al. Mullerian inhibiting substance as a marker for Ovarian sex-cord tumor. N Engl J Med. 1992;326:466–71. doi: 10.1056/NEJM199202133260707. [DOI] [PubMed] [Google Scholar]
- 5.Puls LE, Hamous J, Morrow MS, Schneyer A, MacLaughlin DT, Castracane VD. Recurrent ovarian sex cord stromal tumor with annular tubules. Tumor marker and chemotherapy experience. Gynecol Oncol. 1994;54:396–401. doi: 10.1006/gyno.1994.1232. [DOI] [PubMed] [Google Scholar]
- 6.Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992-1999. Gynecol Oncol. 2005;97:519–23. doi: 10.1016/j.ygyno.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Lele SM, Sawh RN, Zaharopoulos P, Adesokan A, Smith M, Linhart JM, et al. Malignant ovarian sex cord tumor with annular tubules in a patient with Peutz-Jeghers syndrome: A case report. Mod Pathol. 2000;13:466–70. doi: 10.1038/modpathol.3880079. [DOI] [PubMed] [Google Scholar]


