Abstract
The occurrence of intact sterols has been restricted to immature Cretaceous (~125 Ma) sediments with one report from the Late Jurassic (~165 Ma). Here we report the oldest occurrence of intact sterols in a Crustacean fossil preserved for ca. 380 Ma within a Devonian concretion. The exceptional preservation of the biomass is attributed to microbially induced carbonate encapsulation, preventing full decomposition and transformation thus extending sterol occurrences in the geosphere by 250 Ma. A suite of diagenetic transformation products of sterols was also identified in the concretion, demonstrating the remarkable coexistence of biomolecules and geomolecules in the same sample. Most importantly the original biolipids were found to be the most abundant steroids in the sample. We attribute the coexistence of steroids in a diagenetic continuum -ranging from stenols to triaromatic steroids- to microbially mediated eogenetic processes.
Sterols form a specific group of triterpenoid biomolecules, generally abundant in eukaryotes where they fulfill various vital functions, including stabilization of cell membranes, signal messaging and serving as precursors to e.g. vitamins and hormones. Sterols, usually with 26 to 30 carbon atoms, possess specific structural features restricted to groups of organisms1. These biomolecules after senescence from aquatic producers undergo rapid re-mineralization under aerobic conditions in the upper water column. Only a small portion of the intact sterols produced in the euphotic zone endure eogenesis (earliest diagenesis), where microbially mediated transformations effectively yield geomolecules2,3,4,5,6. These compounds can then be related back to their natural product sterol precursors and are more stable in the geologic record. The presence of biological sterols in the rock record is limited to areas of low geothermal gradients and their preservation is enhanced by anaerobic conditions during their deposition and subsequent diagenesis, in particular, early sulfurization and reduction mediated by sulfur species7,8. Intact biological sterols have been observed at trace level concentrations in thermally immature marine shales9 as old as the Upper Albian (~120 Ma). In addition dinosterol and a 24-methylsterol have been reported from sediments of presumed late Jurassic age10. In these sediments original biolipids co-occur with a limited suite of their diagenetic derivatives, possibly due to incomplete degradation of lipids in the water column under high productivity conditions in the presence of selective microbial communities, such as sulfate reducers9,10.
Steroids are often recorded in petroleum as saturated and aromatic steroidal hydrocarbons and are associated with a series of complex transformations occurring during diagenesis and eventually catagenesis. The transformation of sterols during eogenesis is controlled by microbial activity and low temperature physiochemical reactions, involving e.g.: stanols dehydration, sterenes isomerization, diasterenes and monoaromatic steranes formation and subsequent isomerization2,3,4. Eogenetic transformation of steroids is governed by the corresponding environmental conditions, temperature and availability of microbes and catalysts (e.g. clays and/or reduced sulfur). Additional catagenetic alteration of steroids is then attributed to thermodynamically driven abiotic physicochemical reactions with increasing temperature, causing complete aromatization, isomerization and cracking of steroids2,3,4,10. At this stage functionalized steroids are expected to be completely transformed to a more stable form, thus the co-existing of sterols and their intermediate diagenetic products can only occur in immature sediments when incomplete microbial degradation has occurred2,4.
Recently, exceptional low thermal maturity steranes have been reported in a well preserved crustacean fossil, within a carbonate concretion from the Gogo Formation, a Devonian inter-reef deposit of the Canning Basin from the north of Western Australia11,12. The remarkable degree of organic matter preservation at the time of deposition of the crustacean was attributed to the occurrence of persistent euxinic conditions in the photic zone (PZE) prevailing in the ancient sea preventing aerobic degradation processes. These conditions were supported by an active consortium of sulfate reducing bacteria promoting early encapsulation of the biomass facilitating the formation of the carbonate concretion. Here we report even more outstanding preservation of biomolecules due to the observation of intact sterols in the fossilized crustacean, which are the most abundant components over other steroidal hydrocarbons (i.e. geomolecules). This is the first reported occurrence of intact biolipids co-existing with a suite of intermediate diagenetic and catagenetic counterparts preserved in Paleozoic sediments. The consecutive and complex transformations during diagenesis and catagenesis are thought to prevent the parallel occurrence of the most extreme end-members of the steroid pathway, such as functionalized steroids along with fully aromatized counterparts. Our observations challenge this paradigm and point to microbially mediated processes yielding a variety of steroids without thermal overprinting after 380 Ma of their deposition.
Results
A carbonate concretion containing a crustacean in its interior has been previously analyzed showing exceptional organic matter preservation, including low thermal maturity biomarker distribution associated with the fossilized crustacean's soft tissue11. Cholestane was reported to be the most dominant biomarker in this fossil and its presence was attributed to diagenetic-derivatives of cholesterol, the most abundant sterol in living crustaceans11,13. Isomerization of steranes at positions C-5, C-14 and C-17 as well as at the chiral centre at C-20 is concordant with the low thermal maturity of the sample investigated (20S/(20S + 20R) < 0.20). The isomerization of hopanes and hopenes in the sample11 indicates slightly higher thermal maturity with side chain isomerization at the C-22 chiral centre reaching unity for S and R stereoisomers.
Due to the low thermal maturity of the sample naturally occurring sterols were still present and were identified along with a suite of their diagenetic products which include stanols, sterenediols, stenol ketones, stanones, sterenes, diasterenes, diasteranes, C-ring monoaromatic and triaromatic steroids (see supplementary Figs. S1–S4 online); also intact straight chain fatty acids (C16-18 and C28,30) and alcohols (C28,30) were preserved (Figs. 1 and 2). All the steroids identified (Supplementary figures for detailed identification) are indigenous to the fossil and concretion and coexist, thus reflecting a diagenetic continuum. The mixture of steroids found in the sample corroborates the complex sequential biochemical transformation undergone by sterols during eogenesis2,3,4,5,6,9. The co-existence of 70 different steroidal compounds (Table 1) including fully aromatized steroids together with their biological precursors, exclusively found in living organisms, represents the oldest and most extensive sedimentary anachronism reported to date, challenging the paradigm that progressive steroid late dia-/catagenesis is only controlled by thermal maturation. Exceptional preservation may add a new facet to the application of steroid based thermal maturity ratios in petroleum exploration and in reconstruction of thermal histories of sedimentary basins. Presently, co-occurrence of immature and mature biomarker signals has been attributed to i) incorporation of immature biomolecules into migrating oils14, ii) admixture of reworked mature organic matter to immature sediments15, or charging of petroleum reservoirs from source rocks of varying maturities16.
Figure 1.
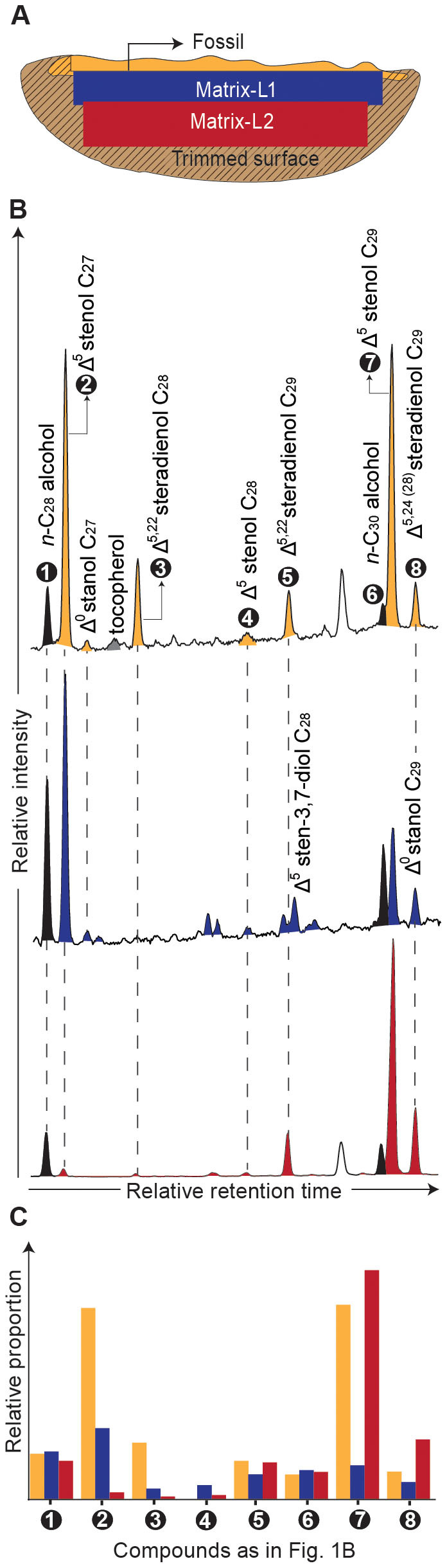
(A) Calcareous concretion containing a well-preserved fossil from the Devonian Gogo Formation. The concretion was split into three layers concentrically away from the nucleus. (B) Partial chromatogram of the free alcohol fraction (as trimethylsilyl-ether derivatives) from the three layers depicting the first occurrence of intact sterols preserved in Paleozoic strata (see Table 1 and Supplementary Figs. S6–8 for detailed identification). (C) Distribution of sterols normalized to the average of C28 and C30 free n-alcohols reveals a dominance of C27-stenols in the fossil layer ascribed to a crustacean input and decreasing proportions towards the matrix, showing elevated C29-stenols derived from algal input.
Figure 2.
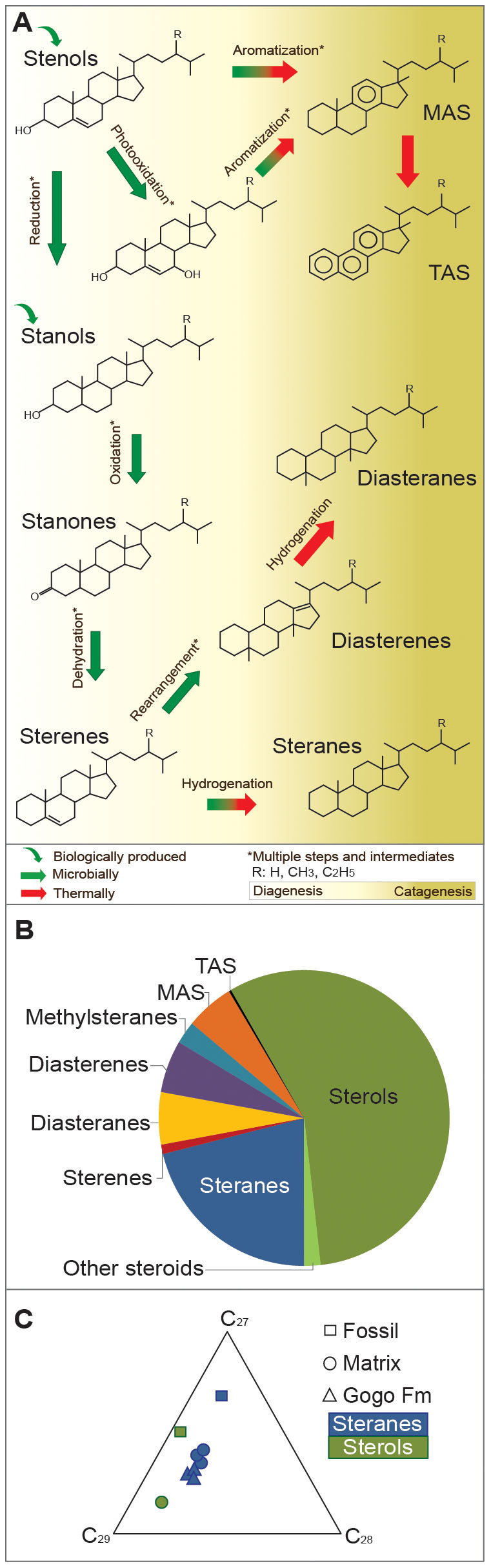
(A) A suite of steroids coexisting in one fossil corresponds to the assemblage of steroids generated by diagenetic (pale yellow background) and catagenetic (dark yellow background) stages of the evolutionary pathways proposed by Mackenzie et al. (3, 4). A total of 70 individual steroids, including stenols, steradienols, stanols, stanones, sterenes, steradienes, diasterenes, diasteranes, mono- and triaromatic steroids, as well as 4-methyl substituted analogues (see Table 1 for quantification of steroids) occur in parallel. (B) Relative proportion of compound classes within the fossil is dominated by sterols and steranes, representing the bio- and geospheric end-members of a diagenetic sequence, respectively. (C) Ternary diagram of sterols (green) and steranes (blue) differentiate the fossil from the concretion matrix and the host rock. The C27 dominance in the fossil layer is attributed to Crustacean tissue, whereas the matrix and host rock represent common algal input(s).
Table 1. Steroid compounds identified in the fossil layer based on their relative elution order and comparison of mass spectra with literature data (Tr: Traces). Peak numbers refer to supplementary figures available online.
| Compound class | Peak N | Identification | Concentration (ppb) | C27/C29 |
|---|---|---|---|---|
| Steranes | 1 | 5α,14α,17α(H) cholestane 20S | 413 | 3.7 |
| 2 | 5α,14β,17β(H) cholestane 20R | 88 | ||
| 3 | 5α,14β,17β(H) cholestane 20S | 74 | ||
| 4 | 5α,14α,17α(H) cholestane 20R | 1503 | ||
| 5 | 5α,14α,17α(H) 24-methylcholestane 20S | 26 | ||
| 6 | 5α,14β,17β(H) 24-methylcholestane 20R | 37 | ||
| 7 | 5α,14β,17β(H) 24-methylcholestane 20S | Tr | ||
| 8 | 5α,14α,17α(H) 24-methylcholestane 20R | 154 | ||
| 9 | 5α,14α,17α(H) 24-ethylcholestane 20S | 70 | ||
| 10 | 5α,14β,17β(H) 20R + 5β,14α,17α(H) 24-ethylcholestane | 131 | ||
| 11 | 5α,14β,17β(H) 24-ethylcholestane 20S | Tr | ||
| 12 | 5α,14α,17α(H) 24-ethylcholestane 20R | 344 | ||
| Sterene | 13 | Δ5 cholestene | 112 | 4.0 |
| 14 | Δ5 24-ethylcholestene | 28 | ||
| Diasteranes | 15 | 13β,17α(H) diacholestane 20S | 127 | 4.6 |
| 16 | 13β,17α(H) diacholestane 20R | 138 | ||
| 17 | 13α,17β(H) diacholestane 20S | 40 | ||
| 18 | 13α,17β (H) diacholestane 20R | 39 | ||
| 19 | 13β,17α(H) 24-methyldiacholestane 20S | 53 | ||
| 20 | 13β,17α(H) 24-methyldiacholestane 20R | 56 | ||
| 21 | 13α,17β (H) 24-methyldiacholestane 20S | 45 | ||
| 22 | 13α,17β (H) 24-methyldiacholestane20R | 35 | ||
| 23 | 13β,17α(H) 24-ethyldiacholestane 20S | 169 | ||
| 24 | 13β,17α(H) 24-ethyldiacholestane 20R | 75 | ||
| Diasterenes | 25 | 10α, Δ13(17) diacholestene 20S | 203 | 3.6 |
| 26 | 10α, Δ13(17) diacholestene 20R | 230 | ||
| 27 | 10α, Δ13(17) 24-methyldiacholestene 20R | 229 | ||
| 28 | 10α, Δ13(17) 24-ethyldiacholestene 20S | Tr | ||
| 29 | 10α, Δ13(17) 24-ethyldiacholestene 20R | 120 | ||
| 4-methylsteranes | 30 | 5α,14β,17β(H) 4α-methylcholestane 20S | 27 | n/a |
| 31 | 5α,14β,17β(H) 4α-methylcholestane 20R | Tr | ||
| 32 | 5α,14α,17α(H) 4α-methylcholestane 20S | Tr | ||
| 33 | 5α,14α,17α(H) 4α-methylcholestane 20R | 106 | ||
| 34 | 5α,14β,17β(H) 4α-methyl 24-ethylcholestane 20S | Tr | ||
| 35 | 5α,14β,17β(H) 4α-methyl 24-ethylcholestane 20R | 38 | ||
| 36 | 5α,14α,17α(H) 4α-methyl 24-ethylcholestane 20S | 86 | ||
| 37 | 5α,14α,17α(H) 4α-methyl 24-ethylcholestane 20R | Tr | ||
| C-ring monoaromatic steroid | 38 | C21 5α, 10β(CH3) | 41 | 2.2 |
| 39 | C22 5α, 10β(CH3) | 45 | ||
| 40 | C27 5β, 10β(CH3) 20S | 72 | ||
| 41 | C27 5β, 10β(CH3) 20R | 43 | ||
| 42 | C27 5α, 10β(CH3) 20S | Tr | ||
| 43 | C28 5β, 10β(CH3) 20S | 170 | ||
| 44 | C27 5α, 10β(CH3) 20R | 78 | ||
| 45 | C28 5α, 10β(CH3) 20S | 99 | ||
| 46 | C28 5β, 10β(CH3) 20R | 71 | ||
| 47 | C29 5β, 10β(CH3) 20S | Tr | ||
| 48 | C29 5α, 10β(CH3) 20S | 44 | ||
| 49 | C29 5α, 10β(CH3) 20SC28 5α, 10β(CH3) 20R | 41 | ||
| 50 | C29 5β, 10β(CH3) 20R | Tr | ||
| 51 | C29 5α, 10β(CH3) 20R | 33 | ||
| Triaromatic steroid | 52 | C26 20S | Tr | 0.5 |
| 53 | C26 20R | Tr | ||
| 54 | C27 20S | Tr | ||
| 55 | C28 20S | Tr | ||
| 56 | C27 20R | Tr | ||
| 57 | C28 20R | Tr | ||
| Functionalized steroids | 58 | cholest-5-en-3β-ol | 2829 | n/a |
| 59 | 24-methylcholest-5-en-3β-ol | 590 | ||
| 60 | 24-ethylcholest-5-en-3β-ol | 4281 | ||
| 61 | cholest-5-en-3β,7α-diol | Tr | ||
| 62 | 24-methylcholest-5-en-3β,7α-diol | Tr | ||
| 63 | 24-ethylcholest-5-en-3β, 7α-diol | Tr | ||
| 64 | Cholest-5-en-3β-ol-7-one | Tr | ||
| 65 | 24-methylcholstenol (unknown isomer) | Tr | ||
| 66 | 24-methylcholesta-5,22-dien-3β-ol | Tr | ||
| 67 | 24-ethylcholesta-5,22-dien-3β-ol | Tr | ||
| 68 | 24-ethylcholesta-5,24(28)-dien-3β-ol | Tr | ||
| 69 | 5α-cholestan-3β-ol | Tr | ||
| 70 | 5α24-ethylcholestan-3β-ol | Tr | ||
| Other compounds | 71 | Tocopherol acetate | Tr | |
| 72 | n-C28 alcohol | Tr | ||
| 73 | n-C29 alcohol | Tr | ||
| 74 | n-C30 alcohol | Tr | ||
| 75 | n-C28 fatty acid | Tr | ||
| 76 | n-C30 fatty acid | Tr |
Exceptional preservation of Devonian sterols occurred in both the fossil and the surrounding layers of the carbonate concretion (Figs. 1A–C). We thus focused our investigation on the fossil layer where the highest steroid concentrations and the largest range of diagenetic derivatives were observed (Table 1). Within the fossil layer sterenes, steranes, diasteranes, diasterenes and monoaromatic steroids with 27 carbon atoms are up to 4 times more abundant than the C29 steroid analogs. The preferential preservation of C27 steroids towards the centre of the concretion, where the fossil is preserved, suggests these originate from the crustacean, resembling the diagenetic products of sterol distributions in living crustaceans, in which cholesterol comprises more than 90% of the total steroids13. However, similar proportion of C27 and C29 Δ5-stenols are present in the proximity of the fossil while in the surrounding matrix the C29 is the most abundant. This distribution of sterols in the sample suggests a combination of sources for the intact biolipids: C27 steroids mainly derived from the fossil tissue (and the Crustacean's dietary products) and C28 and C29 compounds resulting from algae/phytoplankton from the upper water column. The proportion of C28 and C29 is concordant with the stage of algal evolution during the Devonian17,18. This is in agreement with the sterane distribution (C27/C29 < 1) reported for the carbonate matrix surrounding the fossil and the black shale hosting such concretions i.e. the Gogo Formation, dominated by the C29 steranes (see supplementary fig. S5 online)11.
Discussion
The distribution of the two major sterols in the sample, cholest-5-en-3β-ol (δ13C of −26.8‰) and 24-ethylcholest-5-en-3β-ol (δ13C of −30.9‰) and their isotopic disparity confirm the preservation of mixed eukaryotic sources in the fossil layer, i.e. crustacean tissue and that of marine biomass settling through the water column. The toxic water column (i.e. persistent PZE, see above) present at the time11 may have also favored the development of opportunistic blooms of prasinophycean rather than other green algae, as evident by a C28/C29 sterane ratio of > 0.417. The stable isotopic composition of cholestane (δ13C of −30.5‰) in the fossil layer is not fully compatible with an origin exclusive from the co-existing crustacean's cholesterol19. It is thus assumed that the cholestane in the fossil layer based on its carbon isotope signature may have also an algal or zooplanktonic contribution, also supported by the isotopic disparities between isoprenoids and n-alkanes found in the fossil layer11.
Intermediates in the earliest transformation of Δ5-stenols in the water column formed by photo-oxidation, such as cholest-5-en-3β,7α-diol, 24-ethylcholest-5-en-3β,7α-diol, 24-methylcholest-5-en-3β,7α-diol and cholest-5-en-3β-ol-7-one, were also identified in the sample. These compounds are typical rearranged products of 5α-hydroperoxides derived from photo-oxidation (type II) of Δ5-stenols in the euphotic layer of the water column20. Furthermore, trace amounts of tocopherol acetate were identified in the fossil, with the oldest occurrence of tocopherol previously reported for the Cretaceous21. The survival of these highly reactive components can be attributed to a euxinic zone expanding close to the productive surface waters, thus enabling very short transfer times of primary biomass through the oxic water column to the chemocline. Degradation-sensitive biomolecules, when protected within organic debris embedded in the uppermost sediments became rapidly encapsulated within the carbonate concretion and were able to survive some 380 Ma. The difference in the degree of diagenetic transformation between the crustacean and the water column derived sterols is attributed to the excellent preservation of the fossil biomass protected within the crustacean's tissue11.
The parallel occurrence of biolipid stenols with their diagenetic geolipid derivatives including fully aromatized steroids, with the latter present in traces amounts and Δ5-stenols as the dominant compound class, in a concretion that has undergone the same geological history is exceptional. The defunctionalization of sterols to sterenes and their saturated and rearranged counterparts along with the formation of A/B-ring monoaromatic steroids is restricted to the early diagenesis zone, driven by low temperature – microbial reactions2,22,23,24. The later occurring early diagenetic formation of C-ring monoaromatic steroids may be initially microbially mediated but transiently continues into late diagenetic/catagenetic abiotic transformation reactions2,5,25. Depending on the biological precursor present and the depositional conditions prevailing, formation of specific metastable intermediates is favored during earliest diagenesis. In the Gogo concretion the lack of A-ring monoaromatic steroids and B-ring monoaromatic anthrasteroids, spirosterenes and of their presumed precursors, the 3,5-steradienes or 5,7-steradienes24 is attributed to the absence of suitable biological stenols. Although being intermediates in the steroid diagenetic continuum, monoaromatic A/B-ring steroids have been reported from sediments as old as the Cretaceous4,22,23 and spirosterenes in sediments dating to the Malmian stage (148 Ma) of the Jurassic26. The occurrence of catagenetically formed C-ring monoaromatic steroids and triaromatic steroids, however, is not stratigraphically restricted with frequent reports of the stable products in Proterozoic sediments and oils5. In contrast to early diagenesis, the full aromatization and isomerization of chiral centres in steranes and diasteranes are products of thermodynamically controlled physiochemical reactions during latest diagenesis and catagenesis2,3. The fully aromatized steroids are considered to be of exclusively catagenetic origin3,5 but early microbial aromatization of triterpenoids has been reported to occur widely in natural environments. Different aromatization pathways have been formulated for higher plant triterpenoids27 and hopanoic pentacyclic triterpenoids28,29. Here we postulate that eogenetic aromatization processes involving sterols to form triaromatic steroids (Fig. 2A) are also feasible. Disproportionation of hydrogen upon diagenetic processes within the concretion either favored the saturation of sterenes or the progressive desaturation of aromatic steroids.
The burial history of the Gogo Formation excludes temperatures in the catagenesis zone12 and hence indicates structural rearrangement of steranes to form diasteranes and triaromatic steroids in a low thermal diagenetic regime, prior to the oil window. Abiotic mechanisms operating at low temperature cannot be excluded, especially when an active sulfur cycle was present at the time of preservation, in which a consortium of sulfate reducing and green sulfur bacteria existed in a H2S-rich environment11. Evidence of anoxic-euxinic conditions has been proven for this sample, and natural vulcanization has also occurred by early sulfurization of e.g. sterols and isorenieratene derivatives previously reported in the fossil layer11. Elevated microbial activity along with abiotic sulfurization8,30 and non-biological hydrogenation7 favored the preservation of abundant organic matter at early stages of diagenesis playing an important role in the reduction pathway of steroids in an oxygen depleted environment.
The co-occurrence of biomolecules and geomolecules in sediments affords extremely specific prerequisites, not only ensuing unique conditions during primary eogenesis but also after sediment emplacement in a continuation of strictly anaerobic conditions (persistent PZE) during the entire geological history. In addition, it seems to be an essential condition that anaerobic microbial processes within the lower water column and sediment may stimulate the formation of geomolecules formerly ascribed to abiotic thermally governed processes. Under certain conditions the established concept of diagenesis versus catagenesis as such is not applicable. Several lines of evidence provided here indicate that under exceptional conditions concretions are able to preserve biomolecules at unprecedented levels, opening a new window of opportunity to study the distributions of biomolecules in deep time and thus offering prospects in improving our understanding of organismic evolution and past environmental conditions.
Methods
Sample collection and preparation
The carbonate concretions used for this contribution was collected from a field trip to the Canning Basin, North of Western Australia (See details by Melendez et al., 2013). The sample was found weathering out of the rarely exposed basinal black shales in the Paddy's Valley, an extremely arid and remote location north-west of the basin. The concretion contains a well preserved invertebrate which based on chemo-taxonomical properties was identified as a crustacean11.
All the exposed surfaces of the concretion were trimmed off (ca. 10 mm) and slices orientated parallel to the fossil were taken (Fig. 1A). The first slice (i.e. the fossil layer) contains most of the crustacean tissue. Sequential layers (Matrix-L1 and Matrix-L2) were also cut from the carbonate matrix (Fig. 1A).
Each layer was carefully washed with deionized water in an ultrasonic bath (10 min) and dried overnight (40°C). Further external ultrasonic washes were made using dichloromethane (DCM) and methanol (7:3) in triplicate. Cleaned samples were crushed and ground in a zirconium mill. In between each sample the mill was cleaned with solvents and annealed quartz. Organic solvent extracts were obtained by Soxhlet extraction for 72 hours with DCM-Methanol (9:1; v/v) in a pre-extracted cellulose thimble. Each extract was separated into 5 fractions by a small chromatography column (5.5 cm length × 0.5 cm i.d.) packed with activated silica gel (120°C, 8 hour). Aliphatic hydrocarbons were eluted with 1.5 dead volumes (DV) of n-hexane, aromatic hydrocarbons in 2 DVs of 4:1 n-hexane:DCM, ketones and fatty acid methyl esters (FAMEs) in 2DVs of DCM, alcohols in 2 DVs of 4:1 DCM : ethyl acetate and the polar fraction eluted with 2 DVs of DCM : methanol (7:3). Derivatization was conducted on aliquots of the latter 3 fractions using bis(trimethylsilyl)-trifluoroacetamide (BSTFA, 25 μL) and anhydrous pyridine (25 μL). The mixture was heated up to 70°C on a sand bath for 20 minutes and immediately after cooling analyzed by Gas Chromatography-Mass Spectrometry (GC-MS). Procedural blanks were performed to monitor laboratory contamination.
Semi-quantitative analyses were performed on the total lipid extracts, separated fractions and derivatized aliquots by GC-MS using a Hewlett Packard 6890 gas chromatograph (GC) interfaced to a Hewlett Packard 5973 mass selective detector (MSD). The GC-MS was operated in a pulsed splitless mode; the injector was at 320°C and fitted with a DB-5 capillary column (60 m × 0.25 mm i.d. × 0.25 μm film thickness). The oven temperature was programmed from 40°C to 325°C (at 3°C/min) with the initial and final hold times of 1 and 50 min, respectively. Ultra high purity helium was used as the carrier gas and maintained at a constant flow of 1.1 mL/min. The MSD was operated at 70 eV and the mass spectra were acquired in full scan mode, m/z 50–700 at ~ 4 scans per second and a source temperature of 230°C.
A Thermo Finnigan DeltaV mass spectrometer coupled to an Isolink GC (using the same chromatographic conditions as in the GC-MS analysis) was used to determine the δ13C of selected steroids in the underivatized total extract and alcohol fractions. The δ13C values of the compounds were determined by integrating the ion currents of masses 44, 45 and 46, and are reported in parts per mil (‰) relative to the international Vienna Peedee belemnite (VPDB) standard. Reported values are the average of at least two analyses.
Author Contributions
I.M., K.G. and L.S. designed all the experiments and wrote and reviewed the main manuscript text. I.M. performed all the experiments and prepared figures.
Supplementary Material
Supplementary figures
Acknowledgments
Grice and Melendez thank the Australian Research Council (ARC) for a Queen Elizabeth II Discovery grant (awarded to Grice; “Characteristics of organic matter formed in toxic, sulfide-rich modern and ancient sediments”), and for Melendez's Ph.D. stipend. Melendez acknowledges Curtin University for a fee waiver. Grice acknowledges the ARC, John de Laeter Centre and The Institute for Geoscience Research for infrastructure to perform the research. Schwark acknowledges DAAD support for a sabbatical at WA-OIGC, Curtin University under DAAD-ATN Grant No. 53430494. S. Tulipani is thanked for providing a reference sample of the Gogo Formation shale, K. Trinajstic for providing the concretion and G. Chidlow for technical support.
References
- Volkman J. K. A review of sterol markers for marine and terrigenous organic matter. Organic Geochemistry 9, 83–99 (1986). [Google Scholar]
- Mackenzie A. S., Brassell S. C., Eglinton G. & Maxwell J. R. Chemical fossils: the geological fate of steroids. Science 217, 491–504 (1982). [DOI] [PubMed] [Google Scholar]
- Mackenzie A. S., Lamb N. A. & Maxwell J. R. Steroid hydrocarbons and the thermal history of sediments. Nature 295, 223–226 (1982). [Google Scholar]
- Brassell S. C. et al. Isomerisation, rearrangement and aromatisation of steroids in distinguishing early stages of diagenesis. Organic Geochemistry 6, 11–23 (1984). [Google Scholar]
- Peters K. E., Walters C. C. & Moldowan J. M. The Biomarker Guide. 1132 (Cambridge University Press, 2005). [Google Scholar]
- Gagosian R. B., Smith S. O. & Nigrelli G. E. Vertical transport of steroid alcohols and ketones measured in a sediment trap experiment in the equatorial Atlantic Ocean. Geochimica et Cosmochimica Acta 46, 1163–1172 (1982). [Google Scholar]
- Hebting Y. et al. Biomarker Evidence for a Major Preservation Pathway of Sedimentary Organic Carbon. Science 312, 1627–1631 (2006). [DOI] [PubMed] [Google Scholar]
- Adam P. A., Schneckenburger P. S., Schaeffer P. S. & Albrecht P. A. Clues to early diagenetic sulfurization processes from mild chemical cleavage of labile sulfur-rich geomacromolecules. Geochimica et Cosmochimica Acta 64, 3485–3503 (2000). [Google Scholar]
- Comet P. et al. Lipids of an Upper Albian limestone, Deep Sea Drilling Project Site 465. Init. Rep. DSDP 62, 923–937 (1981). [Google Scholar]
- Brassell S. C., Eglinton G. & Howell V. J. Palaeoenvironmental assessment of marine organic-rich sediments using molecular organic geochemistry. Geological Society, London, Special Publications 26, 79–98 (1987). [Google Scholar]
- Melendez I. et al. Biomarkers reveal the role of photic zone euxinia in exceptional fossil preservation: An organic geochemical perspective. Geology 41, 123–126 (2013). [Google Scholar]
- Playford P. E., Hocking R. M. & Cockbain A. E. Devonian Reef Complexes of the Canning Basin, Western Australia. Geological Survey of Western Australia Bulletin 145, 444 (2009). [Google Scholar]
- Kanazawa A. Sterols in marine invertebrates. Fisheries Science 67, 997–1007 (2001). [Google Scholar]
- Curiale J. A. A review of the occurrences and causes of migration-contamination in crude oil. Organic Geochemistry 33, 1389–1400 (2002). [Google Scholar]
- Rowland S. J. & Maxwell J. R. Reworked triterpenoid and steroid hydrocarbons in a recent sediment. Geochimica et Cosmochimica Acta 48, 617–624 (1984). [Google Scholar]
- Leythaeuser D., Keuser C. & Schwark L. Molecular memory effects recording the accumulation history of petroleum reservoirs: A case study of the Heidrun Field, offshore Norway. Marine and Petroleum Geology 24, 199–220 (2007). [Google Scholar]
- Grantham P. J. & Wakefield L. L. Variations in the sterane carbon number distributions of marine source rock derived crude oils through geological time. Organic Geochemistry 12, 61–73 (1988). [Google Scholar]
- Schwark L. & Empt P. Sterane biomarkers as indicators of palaeozoic algal evolution and extinction events. Palaeogeography, Palaeoclimatology, Palaeoecology 240, 225–236 (2006). [Google Scholar]
- Grice K. et al. Effects of zooplankton herbivory on biomarker proxy records. Paleoceanography 13, 686–693 (1998). [Google Scholar]
- Rontani J.-F. Visible light-dependent degradation of lipidic phytoplanktonic components during senescence: a review. Phytochemistry 58, 187–202 (2001). [DOI] [PubMed] [Google Scholar]
- Dumitrescu M. & Brassell S. C. Biogeochemical assessment of sources of organic matter and paleoproductivity during the early Aptian Oceanic Anoxic Event at Shatsky Rise, ODP Leg 198. Organic Geochemistry 36, 1002–1022 (2005). [Google Scholar]
- Hussler G., Chappe B., Wehrung P. & Albrecht P. C27-C29 ring A monoaromatic steroids in Cretaceous black shales. Nature 294, 556–558 (1981). [Google Scholar]
- Hussler G. & Albrecht P. C27-C29 Monoaromatic anthrasteroid hydrocarbons in Cretaceous black shales. Nature 304, 262–263 (1983). [Google Scholar]
- Schüpfer P., Finck Y., Houot F. & Gülaçar F. O. Acid catalysed backbone rearrangement of cholesta-2,4,6-triene: On the origin of ring A and ring B aromatic steroids in recent sediments. Organic Geochemistry 38, 671–681 (2007). [Google Scholar]
- Riolo J., Hussler G., Albrecht P. & Connan J. Distribution of aromatic steroids in geological samples: their evaluation as geochemical parameters. Organic Geochemistry 10, 981–990 (1986). [Google Scholar]
- Schwark L., Vliex M. & Schaeffer P. Geochemical characterization of Malm Zeta laminated carbonates from the Franconian Alb, SW-Germany (II). Organic Geochemistry 29, 1921–1952 (1998). [Google Scholar]
- Le Milbeau C., Schaeffer P., Connan J., Albrecht P. & Adam P. Aromatized C-2 Oxygenated Triterpenoids as Indicators for a New Transformation Pathway in the Environment. Organic Letters 12, 1504–1507 (2010). [DOI] [PubMed] [Google Scholar]
- Trendel J. M. et al. Identification of des-A-triterpenoid hydrocarbons occurring in surface sediments. Tetrahedron 45, 4457–4470 (1989). [Google Scholar]
- Wolff G. A., Trendel J. M. & Albrecht P. Novel Monoaromatic triterpenoid hydrocarbons occuring in sediments. Tetrahedron 45, 6721–6728 (1989). [Google Scholar]
- Kohnen M. E. L., Sinninghe Damsté J. S., Baas M., Dalen A. C. K.-V. & de Leeuw J. W. Sulphur-bound steroid and phytane carbon skeletons in geomacromolecules: Implications for the mechanism of incorporation of sulphur into organic matter. Geochimica et Cosmochimica Acta 57, 2515–2528 (1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures


