Abstract
Total capsaicins are extracted from 2 mL aliquots of serum or plasma using methyl-isobutyl ketone, evaporation of the extract to dryness and reconstitution with 200 μL of acetonitrile. The HPLC mobile phase is 40:60 water:acetonitrile. The absorbance of the eluent is monitored at 205 nm. Standardisation uses a known mixture of pure capsaicin and dihydrocapsaicin. Accuracies are 98.9 and 100.6 % for capsaicin and dihydrocapsaicin respectively. Inter batch reproducibility for both is 15 %. The limits of detection are 2.6 and 3.8 ng/mL for capsaicin and dihydrocapsaicin respectively. Analyses of sera obtained previously from human subjects who had eaten chilli containing meals showed that in those that absorbed capsaicins (N = 30) then the median, mean and SD of their serum capsaicin were: 13.4, 18.9 and 16.3 ng/mL. The corresponding data for those sera (N = 13) that had measurable levels of dihydrocapsaicin were: 6.9, 7.5 and 3.6 ng/mL. This procedure is suitable for use in prospective studies of the metabolism of orally ingested chilli.
Keywords: HPLC, Capsaicin, Dihydrocapsaicin, Serum levels, Post oral dose
Introduction
There is a longstanding interest in the physiological effects of an oral intake of chillies and there is a body of literature that steers us towards regarding chilli as a ‘nutraceutical’. A ‘nutraceutical’ is a food item in our diet that contains not only the conventional nutrients of proteins, carbohydrates and fats but also chemical compounds that have an association with beneficial physiological or healing effects. A simple example is liquorice which is well known to contain natural homologues of salicylic acid i.e. aspirin like compounds. Hence consumption of this food item could be a ‘natural’ way of reaping the benefits of a daily low oral dose of aspirin namely a reduced tendency toward platelet aggregation in the coronary arteries. A more complex example is the use of colostrum by individuals that have inflammatory bowel disease [1]. It is recognised that colostrum confers its palliative effect in these patients via its natural content of epidermal growth factor which in turn promotes healing of the inflamed lesions in bowel that characterise this disease.
When considering whether or not herbs and spices have nutraceutical properties it is reasonable to favour the notion that because we all experience strong taste and smell sensations of these food items then it is probably these ‘taste’ constituents are the causative factors of their beneficial effects. Of all the herbs and spices, chilli has attracted a good deal of interest to the extent that there is a homeopathic based measurement of its ‘gustatory heat’ in solution—the Scoville Unit [2]. It turns out that the compounds that cause the ‘heat’ in chilli belong to a family of compounds known collectively as the capsaicinoids. When you add to this notion the nutritionists’ and epidemiologists’ tendencies to associate the lower incidence of the diseases of affluence in third world countries with the most noticeable differences between western and third world diets, then it is understandable how chilli in particular has come to the fore as a plausible protective agent against the diseases of western affluence.
To prove the case for or against the beneficial and/or protective effect of a food item scientifically it is first necessary to identify the chemical or chemicals in those food items that are likely to be the vehicles for these effects and then to perform formal dose response experiments as if it were a putative pharmaceutical. Our group has gone along with the generally held opinion that indeed it is the capsaicins in chilli which are the active agents, however, the dose of capsaicin administered in our studies [3–6] have not been quantifiable beyond a dose either of whole chillies or of a chilli containing food item such as chilli sauce. With the commissioning of some HPLC equipment we have been able to quantify the capsaicin contents of various botanical varieties and commercial food item preparations of chillies (unpublished) using simple published methodologies [7, 8]. The next step was to determine if capsaicins administered orally as chilli containing meals do actually appear in the human blood stream and if so would it be possible to quantify the amounts. This paper presents an account of our investigations of these possibilities which we first described in prototype form in 2007 [9].
The literature on the measurement of capsaicins is dominated by works relating to the analysis of chilli plant materials and of the capsicum spray products used by police and correctional services personnel. The works of Reilly et al. [10, 11] stood out from this in that their group have published extensively on the chemistry and synthesis of capsaicins, the likely metabolites of natural capsaicins in mammalian systems and an HPLC MS hyphenated technique for measuring capsaicins in human blood samples. We did not have access to comparable instrumentation as used by that group but the HPLC column manufacturer, Agilent, had a Technical Method Sheet for capsaicins using their Luna C18 column. We adopted the approach that we would use solvent extraction as per the Reilly et al. [12] group and the HPLC elution solvents as suggested by Agilent. As a result we have arrived at an analytical methodology which can measure capsaicin and dihydrocapsaicin in human serum or plasma at ng/mL levels.
Materials and Methods
Equipment
HPLC Column: Phenomenex Luna 3 micron C18(2), 150 X 3 mm, reversed phase column (Lane Cove, Australia)
Phenomenex KJO-4282 Security Guard Cartridge (Lane Cove, Australia)
Hamilton syringe, 100 μL, glass ‘Gas Tight’ model with stainless steel plunger (Hamilton Company, Reno, NV)
Qualtex Magnetic Stirrer (Watson Victor, Melbourne, Australia)
Model B100 S/E vacuum pump capable of a negative pressure of -750 bar (Charles Austen Pumps, Weybridge, UK)
Agilent Model 1100 Integrated HPLC system (Agilent Technologies, Santa Clara, SA)
Agilent ChemStation software Revision A.10.02 [1757] installed on a Dell Inspiron 1150 computer (Agilent Technologies, Santa Clara, SA)
Reagents
Acetonitrile: J T Baker Ultra Gradient HPLC Grade Acetonitrile (Mallinkrodt-Baker, Devester, Holland)
Methanol: J T Baker Methanol (Baker HPLC Analyzed) (Mallinkrodt-Baker, Devester, Holland)
4-methyl-2-pentanone [Methyl isobutyl ketone, (MIBK)] ACS reagent ≥ 98.5 % (Sigma-Aldrich, St. Louis MO, USA)
Phosphate buffer: pH 7.0: KH2PO4 5.3 g/L, Na2HPO4 8.68 g/L prepared from analytical grade reagents.
(E) capsaicin, (Tocris, Avonmouth, UK)
Dihydrocapsaicin (Fluka) Sigma-Aldrich product 37274
Reverse osmosis water (RO water) (Compass Water Solutions RO 120-0 Reverse Osmosis System, Irvine, CA)
Composition of the Mixed Standard
Capsaicin: 200 ng/mL
Dihydrocapsaicin: 250 ng/mL
Solvent: HPLC grade acetonitrile
Food Items
Chilli Sauce comprising 62 % chilli (predominantly the Habanera botanical variety of chilli plant), water, sugar, salt, vinegar and vegetable gum. One teaspoon of this sauce was approximately equivalent to one fresh chilli (MasterFoods, Wyong, NSW, Australia)
Unbranded ‘Bird’s Eye’ Chilli Powder: obtained from local spice merchant (Birds Eye is a botanical variety of chilli plant)
Serum Sample Preparation Procedure: Extraction with MIBK Followed by HPLC Analysis on an Agilent 1100 Instrumentation
2 mL of serum or plasma sample were pipetted into a 10 mL glass Hach tube. 2 mL phosphate buffer, (pH 7.0), followed by 2 mL methyl isobutyl ketone (MIBK) were added. The tube was then capped and inverted gently two or three times. It was important that at this stage the mixture was not vortex mixed; if it was then there was a serious risk of emulsion formation which would not separate readily at the following centrifugation step. The tube was then placed on a roller mixer at medium speed for 10 min before centrifuging at 1,922 rcf for 20 min at room temperature. All of the organic layer was transferred to a clean 5 mL glass screw top tube using a 2 mL glass graduated pipette. This solvent was evaporated off by placing the tubes into a thermostatically controlled heating block set at 40 °C. To accelerate the evaporation process either gentle vapour suction using a custom made manifold or placement of the rack of tubes in the very front of a fume hood so that the draught was directly across the tops of the open tubes was used. This process took between two and two and half hours depending on the ambient temperature in the laboratory and the airflow velocity across the tops of the open tubes. Once dry the tubes were capped and stored at 4 °C until convenient to run the sample on the HPLC instrument. When required the dried residue was reconstituted with 200 μL of acetonitrile and placed on a roller mixer at medium speed for 10 min
Analysis of Serum Sample Extracts on the Agilent HPLC Instrument
The Agilent Autosampler Injector was programmed to inject 20 μL into the eluent stream. We found that the optimum eluent on this instrumental setup was a 40:60 mixture of RO water:acetonitrile, pumped at the same flow rate of 0.5 mL per minute through a Phenomenex Luna C18(2) column at room temperature. Absorbance of the effluent was monitored at 205 nm and the ChemStation software was used to capture the raw data and perform the retention time and peak area calculations.
Validation by the ‘Method of Standard Additions’
We carried out experiments in which we determined the exact levels of capsaicins in a pooled human serum which had been spiked with natural capsaicins from chilli powder. To spike the serum 200 mg of Birds Eye chilli powder were added to 2 mL serum taken from a blank pooled serum. It was mixed thoroughly and left for 24 h at 4 °C. It was then centrifuged for 10 min at 1,922 rcf and the supernatant removed. The supernatant was filtered through a 0.2 μm syringe filter. 20 μL of this filtered spiked serum was added to 100 mL of pooled blank patient serum. The ‘Method of Standard Additions’ was used to determine the exact capsaicin and dihydrocapsaicin concentrations in 2 mL aliquots of this spiked pool; the additions used were 200 and 400 μL of the “200” Mixed Standard solution. (200 and 400 μL of the “200” Mixed Standard contained 40 and 80 ng of capsaicin and 50 and 100 ng of dihydrocapsaicin). The technique followed for making these additions was critical. We observed that straight addition of a solution of capsaicins in acetonitrile would produce no subsequently measurable capsaicins. We deduced that addition of the acetonitrile had caused micro precipitation of serum proteins which in turn had chelated all the added capsaicin. The only successful technique was to pipette the standard additions solution into the glass tube and then evaporate that to dryness. Serum could then be added to the tube and the serum proteins took up all the dried capsaicins.
Standardisation
All unknown samples were bracketed with paired analyses of the “200” Mixed Standard.
Dose–Physiological Response Trials
The subjects, who had fasted overnight, were given 20 g of Master Foods Chilli Sauce as part of the test meal. Blood samples had been collected at 20, 40, 60, 90 and 120 min post ingestion of the chilli sauce containing test meal.
Results and Discussion
The sample preparation procedure described in the preceding sections was arrived at after extensive experimentation with a range of serum sample preparation techniques. We found that all protein precipitation techniques commonly used in clinical chemistry, and including acetonitrile, methanol and acid alcohol, resulted in total loss of the capsaicins in the supernatant derived from sera with quite elevated additions of capsaicins in solutions. It was only when we used solvent extraction that we were able to detect the capsaicins in the solvent derived layer. Solid phase extraction was excluded because the volume of the eluent associated with those devices was so large that the concentrations of the capsaicins were too low to be practicably measureable without further pre-concentration by evaporation. This would have taken significantly longer than our adopted procedure. It addition we observed that capsaicins avidly bind to plastics and given that all commercial solid phase extraction systems are constructed from plastics we anticipated that recoveries from those eluates would be unpredicatable and/or compromised.
We found that for any of our work to be successful we had to use glassware throughout. Use of conventional disposable plastic pipette tips was only permitted when pipetting solvents or serum/plasma samples. We also concluded that because capsaicins bind so avidly to serum and plasma proteins, it was acceptable practice to store these preanalytical samples in conventional plastic sample tubes and we could safely aliquot them using plastic pipette tips.
We also conducted experiments that had shown that the MIBK extraction was a superior technique to the n-butyl chloride based (NBC) extraction favoured by Reilly’s group [10]. 100 % recoveries of the 2 mL organic phase were possible because of the clarity of the boundary layer and the extraction efficiency of MIBK for capsaicins was found to be exactly equivalent to that of NBC.
Chromatograms Obtained from Serum Samples
Run time was 17 min per extract followed by a ‘cleaning cycle’. The cleaning cycle involved injecting 40 μL of methanol and eluting for 10 min before injecting 40 μL of acetonitrile and eluting for 10 min. The methanol and acetonitrile were placed on the autosampler as ‘dummy’ samples and programmed to just run for 10 min each. With this arrangement we could analyze 1.5 samples per hour.
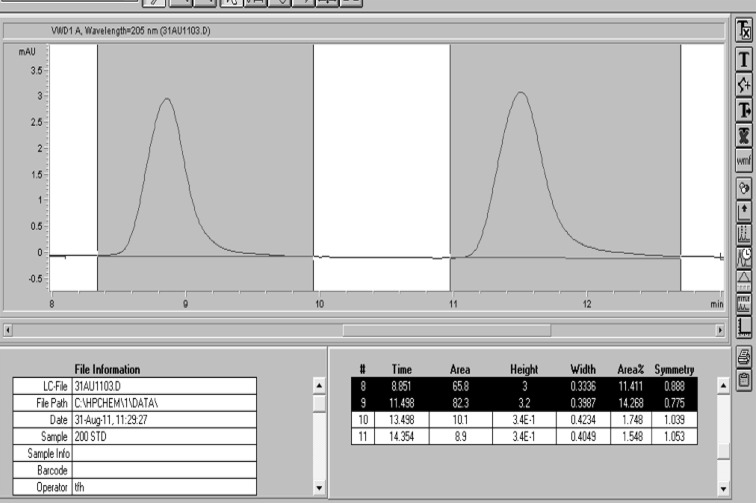
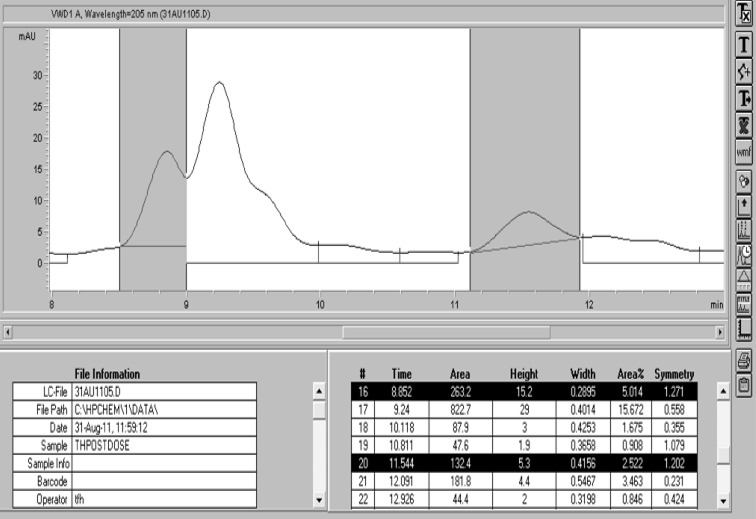
Retention times were 8.74 min for capsaicin (CV% = 2.3 %) and 11.34 min for dihydrocapsaicin (CV% = 2.6 %) measured over 36 injections of the ‘200’ Standard Solution. Typical chromatograms for the Mixed Standard and a serum sample extract are shown in Figs. 1 and 2.
Fig. 1.
Typical chromatogram obtained for the mixed standard (capsaicin = 200 ng/mL, dihydrocapsaicin = 250 ng/mL) when extracted with MIBK and run on the Agilent 1100 instrument with 60:40 acetonitrile:water as the eluant
Fig. 2.
Typical chromatogram obtained for a post chilli dose serum sample when extracted with MIBK and run on the Agilent 1100 instrument with 60:40 acetonitrile:water as the eluent
Validation
Standardisation of HPLC methods is always problematic. Conventionally in HPLC internal standards are added to samples in a known amount and taken through the sample preparation procedure. These internal standards are usually selected not because they are chemically similar to the analytes of interest but because fortuitously they
- (a)
elute within a reasonable timeframe and clear of the peaks of the analytes of interest and
- (b)
are detectable at the same wavelength used for the analytes of interest
We did pilot work with two such candidate internal standards namely oestradiol [13] and 5,5-diphenylhydantoin (Dilantin). We found that the oestradiol was unusable if the serum samples were collected from female subjects. 5,5-diphenylhydantoin was unusable because it was not well resolved in serum samples with high carotenoids. The capsaicin peak became overlapped with the wide bases of the complex of carotenoid peaks that elute within the first 5 min in those samples.
Our solution was to use the Method of Standard Additions described previously in Materials and Methods to calibrate two serum pools that we could then use prospectively in analytical runs of ‘unknowns’.
Standardisation, Intra Batch Accuracy and Reproducibility
Seven different patient pooled blank sera were analysed using the sera calibrated by the Method of Standard Additions as the standards. This calibrated serum was analysed at the beginning and at the end of the batch of spiked patient sera. Each aliquot of the blank serum was spiked with 143 μL of a 1 in 350 dilution of the calibrated serum.
The mean capsaicin concentration in the seven sera was estimated to be 179 ng/mL with a CV% of 9.1 %. The mean dihydrocapsaicin concentration in the seven sera was estimated to be 89 ng/mL with a CV% of 7.4 %.
An important finding of this study was that using either the calibrated serum as the standard or the “200” Mixed Standard, which had also been run during this experiment, produced almost identical results. Because of this it was decided that all further samples could be analysed using direct HPLC analysis of aliquots of the “200” Mixed Standard within each run. The procedure adopted was to perform two HPLC analyses of the “200” Mixed Standard at the beginning of a batch of reconstituted serum extracts and two at the end of the batch. In this way we could detect and compensate for any possible instrumental drift and changes in the background noise. We would use the mean of the four areas under those standard peaks in the calculation of the concentrations in the serum samples.
Inter-Batch Reproducibility
The data from 25 analyses of the mixed standard (capsaicin = 200 ng/mL, dihydrocapsaicin = 250 ng/mL) expressed as peak areas made on 8 different days showed that the inter-batch reproducibilities were 12.1 and 11.1 % respectively for capsaicin and dihydrocapsaicin.
Concentrations of Capsaicinoids in Patient Serum Samples
The Agilent system had the advantages of an automated sample injector and software (ChemStation). Capsaicinoid concentrations in unknown samples in ng/mL were calculated using the formulae:
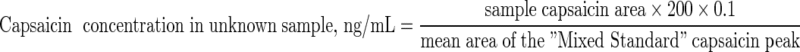 |
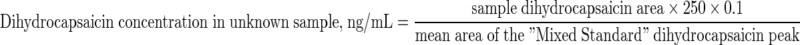 |
Derivation of the Constant ‘0.1’ in the Formulae
2 mL of serum were extracted into the same volume of MIBK, evaporated to dryness then reconstituted into 200 μL of acetonitrile. This meant that the concentration of analytes in that 200 μL of acetonitrile was 10 times higher than in the original serum; 0.2/2 = 0.1.
Limit of Detection
A representative set of results for the mixed standard obtained from eight analytical runs is shown in Table 1. The instrument’s units of peak area were based upon width of the peak in units of time multiplied by the heights of the peak’s profile in absorbance units. We have examined the span of areas in each of the eight runs and taken these as an estimate of the average ‘valley to peak’ baseline noise, 7.5 and 11.0, for capsaicin and dihydrocapsaicin respectively. In terms of concentrations these approximate to 2.6 and 3.8 ng/mL for capsaicin and dihydrocapsaicin respectively. These levels are probably reasonable estimates of the limit of detection limit of the assay on an inter-batch basis. However it is clear that on some runs the HPLC performed considerably better than on others; a common observation in HPLC work. For example for runs 12AUxx and 01SExx the limit of detection would have been some three to more than ten times lower.
Table 1.
Inter-batch reproducibility data from the analyses of the ‘200’ mixed standard
| File # | Peak areas | |
|---|---|---|
| CAP | H2CAP | |
| 27JN1101a | 52.8a | 64.9a |
| 27JN1104a | 48.8a | 64a |
| 27JN1116a | 50.3a | 73.1a |
| 13JY1109 | 54.5 | 71.4 |
| 13JY1112 | 55.6 | 71.2 |
| 13JY1127 | 54 | 68.8 |
| 13JY1130 | 49.3 | 63.8 |
| 14JY1103a | 58.4a | 72.9a |
| 14JY1106a | 54a | 70a |
| 14JY1121a | 54.7a | 72.2a |
| 12AU1101 | 55 | 71.1 |
| 12AU1103 | 54.3 | 71.9 |
| 28AU1101a | 46.5a | 58.2a |
| 28AU1103a | 53.1a | 65.7a |
| 28AU1111a | 55.3a | 72.8a |
| 28AU1114a | 61.5a | 71.7a |
| 31AU1103 | 65.8 | 82.3 |
| 31AU1101 | 65 | 83.1 |
| 31AU1117 | 55.7 | 61.1 |
| 01SE1101a | 69.4a | 79.9a |
| 01SE1103a | 67.2a | 85.4a |
| 02SE1101 | 69.3 | 81.9 |
| 02SE1103 | 69.8 | 88.6 |
| 02SE1114 | 63.1 | 66.9 |
| 02SE1117 | 52.7 | 61.9 |
| Mean Area | 57.44 | 71.79 |
| SD | 6.929 | 7.961 |
| CV% | 12.1 | 11.1 |
a Separation between successive within batch triplets or quadruplets of the mixed standard
CAP capsaicin, H2CAP dihydrocapsaicin
Capsaicin and Dihydrocapsaicin Levels in Sera Collected from Human Subjects Post Ingestion of a Chilli Containing Meal
Our group has had a longstanding interest in the effects of oral intakes of varying amounts of chilli on various physiological responses such as glucose homeostasis. Up until the time of this development work it was not possible to quantitate the dose of capsaicins actually given to subjects, only the grams of chilli sauce added to the meal. Analyses of methanolic extracts of the MasterFoods Chilli Sauce using HPLC and the same buffers, column and wavelength described for the serum samples indicated that the sauce contained 390 and 200 μg/g of capsaicin and dihydrocapsaicin respectively.
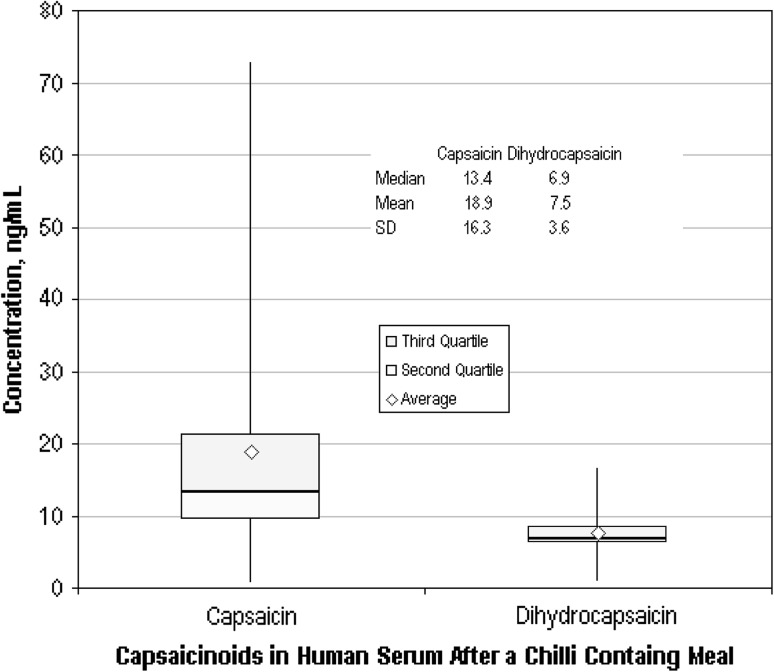
Using these HPLC we have collected data from stored serum samples from the study outlined in 2.6. A significant number of the sera from the blood samples collected during that study had been stored at −80 °C and were available for retrospective analysis. We observed that samples that had measurable capsaicin levels (N = 30), did not always have measurable dihydrocapsaicin (N = 13). This was probably due to that fact that in chillies the level of capsaicin is usually twice that of the dihydrocapsaicin. Hence a low capsaicin level could mean that the corresponding level of dihyrocapsaicin was below our detection limit. Nor could we confidently identify a trend between the time the sample was collected and the corresponding levels of the capsaicinoids. Figure 3 presents the results of the parametric and non-parametric statistical analyses of the pooled data. Another interesting feature of these data was that the ratio of the mean capsaicin level, 18.9 ng/mL, to the mean of the mean dihydrocapsaicin level, 7.5 ng/mL, was 1.94:1. this matched almost exactly the same ratio we had measured in the chilli sauce viz 390:200 μg/g = 1.95:1 (unpublished work). We took this as further evidence that our procedure was successfully measuring the capsaicins derived from the ingested chilli sauce. It also implied that there appeared to be no selective/preferential absorption of the two capsaicin molecules.
Fig. 3.
Box and whisker plots and parametric statistics of the results obtained from the serum samples collected post ingestion of a meal containing 20 g of chilli sauce
Concluding Remarks
This solvent extraction based HPLC procedure has been successful. The success has been due to the 10 times concentrating factor of the solvent extraction step in combination with a modern high performance C18 column, which brought the levels of capsaicins in the HPLC eluent to within the operating range of contemporary high sensitivity UV/Visible detectors. One avenue for possible improvement in terms of sample throughput is to obtain the synthetic internal standard used by Reilly et al. [10, 11] as this would obviate the need for bracketing each lot of samples with the mixed capsaicin dihydrocapsaicin standard. Notwithstanding this, we have concluded that the overall performance characteristics are suitable both in terms of sample throughput and analytical performance. We were confident that it would be suitable for prospective studies of the metabolism of orally ingested chilli containing meals by our group. These studies have the potential to provide in vivo evidence of those purported beneficial effects that up until now have been based largely upon in vitro studies.
Acknowledgments
We wish to acknowledge the ongoing interest in and commentary on this work given by Assoc. Prof. Dominic Geraghty and other members of staff of the School of Human Life Sciences.
Conflict of interest
We hereby declare that none of the authors had any association or affiliation with third parties that had an intellectual property or financial interest in the work described herein. At no stage was the direction of this research work influenced by external third parties. No experiments on animals or humans were performed during this analytical method development study.
Abbreviations
- μL
Microliter
- CV%
Coefficient of variation (expressed as a percentage of the standard deviation)
- HPLC
High pressure liquid chromatography
- HPLC MS
High pressure liquid chromatography mass spectrometry
- MIBK
Methyl-isobutyl ketone
- mL
Millilitre
- NBC
n-Butyl chloride
- ng
Nanogram
- rcf
Relative centrifugal force
- UV
Ultraviolet
Contributor Information
Thomas Hartley, Phone: +61-3-6222 8780, FAX: +61-3-6324-3658, Email: tom.hartley@dhhs.tas.gov.au, Email: thomas.hartley@utas.edu.au.
Brian Stevens, Email: b.stevens@mhri.edu.au.
Kiran D. K. Ahuja, Email: kiran.ahuja@utas.edu.au
References
- 1.Playford RJ, Macdonald CE, Johnson WS. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am J Clin Nutr. 2006;72(1):5. doi: 10.1093/ajcn/72.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Batchelor JD, Jones BT. Determination of the Scoville heat value for hot sauces and chilies An HPLC experiment. J Chem Educ. 2000;77:266. doi: 10.1021/ed077p266. [DOI] [Google Scholar]
- 3.Ahuja KDK, Ball M. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Brit J Nutr. 2006;96:239. doi: 10.1079/BJN20061788. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja KDK, Kunde DA, Ball MJ, Geraghty DP. Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids. J Agric Food Chem. 2006;54:6436. doi: 10.1021/jf061331j. [DOI] [PubMed] [Google Scholar]
- 5.Ahuja KDK, Robertson IK, Geraghty DP, Ball MJ. Effects of chili consumption on postprandial glucose, insulin and energy metabolism. Am J Clin Nutr. 2006;84:63. doi: 10.1093/ajcn/84.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja KDK, Robertson IK, Geraghty DP, Ball MJ. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur J Clin Nutr. 2007;61:326. doi: 10.1038/sj.ejcn.1602517. [DOI] [PubMed] [Google Scholar]
- 7.Edwards SJ, Colquhoun EQ, Clark MG. Levels of pungent principles in chilli sauces and capsicum fruit in Australia. Food Aust. 1990;42:432. [Google Scholar]
- 8.Huang J, Mabury SA, Sagebiel JC. Hot chili peppers : extraction, cleanup and measurement of capsaicin. J Chem Educ. 2000;77:1630. doi: 10.1021/ed077p1630. [DOI] [Google Scholar]
- 9.Hartley T, Stevens B, Ahuja K. Development of an HPLC method for the determination of capsaicins in human plasma. IJCB. 2007;22(Supl):160. [Google Scholar]
- 10.Reilly CA, Crouch DJ, Yost GS, Fatah AA. Determination of capsaiciun, nonivamide and dihydrocapsaicin in blood and tissue by liquid chromatography–tandem mass spectrometry. J Anal Toxicol. 2002;26:313. doi: 10.1093/jat/26.6.313. [DOI] [PubMed] [Google Scholar]
- 11.Reilly CA, Ehlhardt WJ, Jackson DA, Kulanthaivel P, Mutlib AE, Espina RJ, Moody DE, Crouch DJ, Yost GS. Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chem Res Toxicol. 2003;16:336. doi: 10.1021/tx025599q. [DOI] [PubMed] [Google Scholar]
- 12.Phenomenex Application ID No. 1076: Cayenne (Oleoresin capsicum).
- 13.Novakova L, Solich LP, Matysova L, Sicha J. HPLC determination of estradiol, its degradation product and preservatives in new topical formulation Estrogel HBF. Anal Bioanal Chem. 2004;379:781. doi: 10.1007/s00216-004-2532-2. [DOI] [PubMed] [Google Scholar]