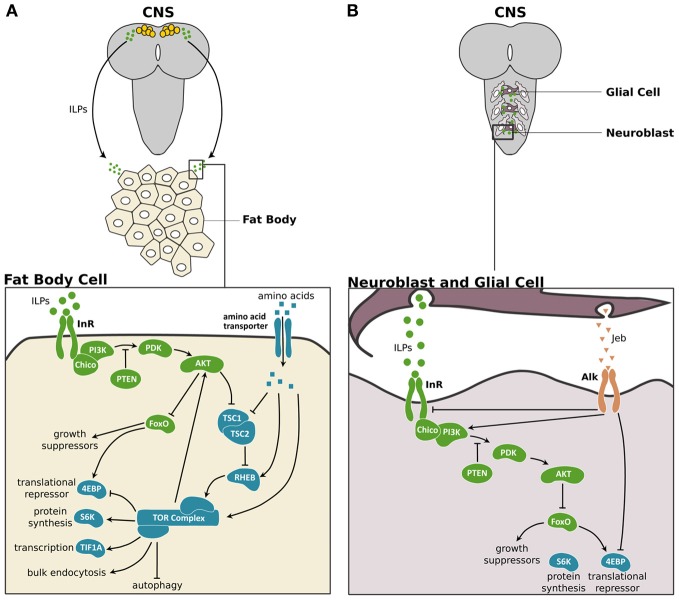
Figure 1.
The IIS/TOR signaling pathway in fat body and the central nervous system of Drosophila. (A) In Drosophila, three of the eight ILPs, ILP2, ILP3, and ILP5 are expressed in a set of neurosecretory cells in the central nervous system (CNS) (Brogiolo et al., 2001; Ikeya et al., 2002). These ILPs activate insulin/insulin-like growth factor signaling (IIS) in peripheral tissues like the fat body cells. In the fat body, the ILPs bind to and activate the Insulin Receptor (InR), which in turn activates Chico, the insulin receptor substrate. This activation initiates a phosphokinase signal transduction cascade that involves phosphatidylinositide 3-kinase (PI3K), the phosphoinositide-dependent protein kinase 1 (PDK) and the protein kinase Akt (Sarbassov et al., 2005). The phosphatase and tensin homolog (PTEN) catalysis the reverse reaction promoted by PI3K, thereby inhibiting the IIS pathway (Goberdhan et al., 1999; Gao et al., 2000). When activated, Akt promotes cell growth by inhibiting the transcription factor Forkhead Box class O (FoxO) (Junger et al., 2003), which activates the 4E-Binding Protein (4EBP), a translational repressor (Miron and Sonenberg, 2001). Furthermore, Akt suppresses the negative regulators of the Target of Rapamycin (TOR) pathway, Tuberous Sclerosis Complex 1 and 2 (TSC1/2) (Gao and Pan, 2001; Gao et al., 2002). TOR activity itself is enhanced by high concentrations of intracellular amino acids (Gao et al., 2002) via the Ras Homolog Enhanced in Brain (RHEB; Garami et al., 2003). TOR activity promotes cell growth by enhancing translation and ribosome biogenesis through inhibition of the 4EBP and activation of the ribosomal protein S6 kinase (S6K). TOR also stimulates rRNA synthesis by activating the Transcriptional Intermediary Factor 1A (TIF-1A) (Hietakangas and Cohen, 2007). Lastly, TOR promotes bulk endocytosis and inhibits autophagy. TOR feeds back on the IIS pathway by regulating Akt in a cell autonomous manner (Hietakangas and Cohen, 2007). (B) In the CNS two ILPs, ILP3 and ILP6, are secreted by surface glia and activate the IIS pathway in the neuroblasts to regulate the growth of this tissue (Chell and Brand, 2010; Sousa-Nunes et al., 2011). The anaplastic lymphoma kinase (Alk) and its ligand, Jelly belly (Jeb) promote growth of neuroblasts in starved larvae (Cheng et al., 2011). Alk promotes CNS growth in starved conditions by suppressing InR and directly stimulating PI3K activity. IIS components are shown in green, TOR pathway components are shown in blue and Jeb/Alk components are shown in orange.