Abstract
Munn et al. made a scientific observation of major biological importance. For the first time they showed that in the mammal the fetus does survive an immune attack mounted by the mother, and that the mechanism responsible for the survival depends on the fetus and placenta 'actively' defending itself from attack by maternal T cells by means of an enzyme indoleamine 2,3-dioxygenase (EC 1.13.11.42) dependent localised depletion of L-tryptophan. These findings raise critical questions for disease and its prevention during human pregnancy. Specifically, the role of this mechanism (discovered in mouse) in the human, and the extent to which defective activation of this process is responsible for major clinical diseases are unknown. Therefore some key facts about this enzyme expressed in the human placenta have been studied in order to test whether Munn et al.'s findings in mouse are met for human pregnancy. This short review attempts to describe our experimental work on human placental indoleamine 2,3-dioxygenase.
Keywords: Human placenta; Indoleamine 2,3-dioxygenase; Pre-eclampsia; Pregnancy; Tryptophan
Introduction
It has been recognised as paradoxical that a genetically disparate mammalian conceptus expressing paternal as well as maternal gene products does not succumb to any maternal immune response during pregnancy. Medawar proposed three possible hypotheses to explain the paradox of maternal immunological tolerance to the fetus [1]: anatomical separation of mother and fetus, antigenic immaturity of the fetus and suppression or modification of the maternal immune system. It appears that the first two proposals cannot explain fetal allograft survival. The maternal fetal interface is not a complete barrier and fetal cells can migrate into the maternal circulation or vice versa [2]. The second explanation is also nearly ruled out since both fetal and placental cells express major histocompatibility complex molecules (MHC) [3]. Major interest has therefore been focused on the third mechanism in which the survival of the allogeneic fetus depends on the fetus and placenta actively defending itself from attack by the cells of the maternal immune system. Munn et al. [4] added indoleamine 2,3-dioxygenase as a new candidate to the list of potential immunosuppressive mechanisms in pregnancy.
The enzyme indoleamine 2,3-dioxygenase (EC 1.13.11.42) is a heme-containing protein that catalyses the oxidative cleavage of the indole ring of tryptophan upon the insertion of two oxygen atoms of molecular oxygen [5]. Indoleamine 2,3-dioxygenase is widely expressed in a variety of tissues of mammals such as rabbit, rat, mouse and humans. One tissue with particularly high activity is the human placenta [6]. Although the precise physiological roles of indoleamine 2,3-dioxygenase is still unknown, the enzyme is induced under pathological conditions including virus infection [7] and parasitic infestation [8], resulting in the rapid degradation of tryptophan to kynurenine in the infected cells. Interferon-γ which exerts potent immunomodulatory and antiproliferative effects strongly induces the expression of the gene coding for indoleamine 2,3-dioxygenase [9]. It is possible to speculate that the inhibitory effect of interferon-γ on intracellular pathogens is, at least in part, attributable to the depletion of the essential amino acid, L-tryptophan following induction of indoleamine 2,3-dioxygenase. In addition to defense against pathogens, this enzyme exerts important immunomodulatory effects. Indoleamine 2,3-dioxygenase suppresses T-cell responses by depleting local L-tryptophan, an essential amino acid for T-cell proliferation and function [10]. Indoleamine 2,3-dioxygenase mediated T-cell suppression plays a crucial role in long term allogeneic organ graft function and in the control of graft-versus-host disease after allogeneic bone marrow transplantation and peripheral blood stem cell transplantation [11].
With regard to the role of placental indoleamine 2,3-dioxygenase Munn et al. [4] formulated the hypothesis that in normal pregnancy expression of this enzyme at the maternal-fetal interface is necessary to prevent immunological rejection of the fetal allograft. To test this hypothesis they exposed pregnant mice (carrying syngeneic or allogeneic fetuses) to 1-methyl-tryptophan, a pharmacological agent that inhibits indoleamine 2,3-dioxygenase activity [12]. They found that T cell-induced rejection of all allogeneic (but not syngeneic) concepti occurred rapidly when pregnant mice were treated with the inhibitor. However fetal allograft rejection was not observed when recombination activating gene 1 (RAG-1) deficient mothers that cannot develop lymphocytes were treated with 1-methyl-tryptophan. They also demonstrated that adoptive transfer of splenocytes from T cell receptor transgenic mice harbouring cohorts of CD8 positive T cells specific for a paternally inherited fetal MHC class I alloantigen caused fetal allograft rejection in these mothers lacking lymphocytes. They suggested T cells are inhibited by a mechanism involving indoleamine 2,3-dioxygenase dependent localised depletion of tryptophan at the site of placentation.
The maternal syndrome of pre-eclampsia is a major complication of human pregnancy with significant morbidity and mortality. Although the aetiology of pre-eclampsia remains unknown, there is substantial evidence that the clinical disease is secondary to abnormalities of placentation [13], with increased oxidative stress [14] and maternal endothelial cell activation and dysfunction [15]. In addition to endothelial dysfunction, systemic activation of maternal inflammatory cell populations has been observed including granulocytes [16], monocytes [17] and even lymphocytes [18]. Recent studies have shown that pre-eclampsia is indeed associated with an inflammatory response similar to that observed in septic patients and is associated with increased production of intracellular reactive oxygen species. However it seems that normal pregnancy is also associated with an inflammatory response albeit less severe [19]. From Munn et al.'s hypothesis that placental indoleamine 2,3-dioxygenase is responsible for suppressing the maternal immune response against the allogenic fetus by depleting tryptophan at the maternal-fetal interface it is possible to suggest that in pre-eclampsia changes in the activity or levels of indoleamine 2,3-dioxygenase in the placenta may be involved in the pathogenesis of this condition.
As mentioned above, Munn et al. [4] made a scientific observation of major biological importance. Their findings raise critical questions for human pregnancy. Specifically, the role of this mechanism (discovered in mouse) in the human, and the extent to which defective activation of this process is responsible for major clinical diseases are unknown. We therefore have studied some key facts about this enzyme expressed in the human placenta in an attempt to test whether Munn et al.'s findings in mouse are met for human pregnancy. In this review, particular focus will be placed on the results of our study on this enzyme in the human placenta.
Localisation and characterisation of human placental indoleamine 2,3-dioxygenase
In human placental tissue indoleamine 2,3-dioxygenase is detectable immunohistochemically from day 6 human blastocysts and thereafter throughout pregnancy in syncytiotrophoblasts, cytotrophoblasts and macrophages in the villous stroma and in the fetal membranes [20]. The results of cellular fractionation suggest that the placental isoform of indoleamine 2,3-dioxygenase is not expressed in the maternal facing brush border membrane of the syncytiotrophoblast but is a cytoplasmic enzyme [21].
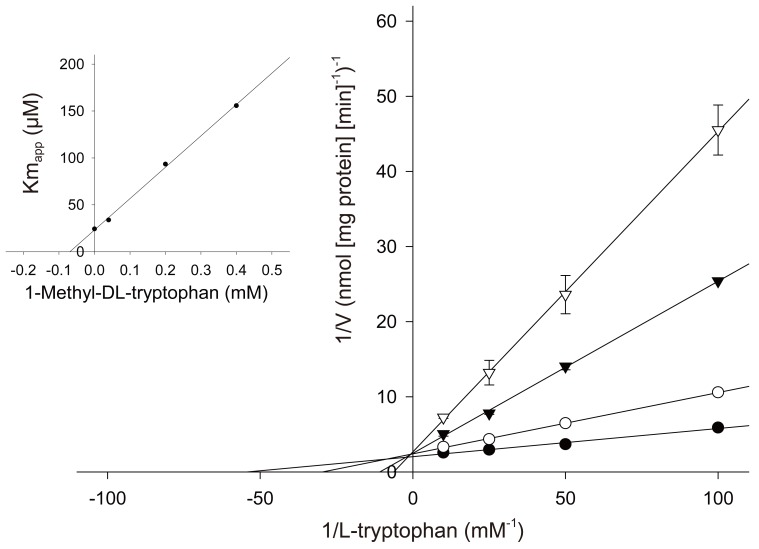
1-Methyl-tryptophan which was used by Munn et al. [4] for inhibiting indoleamine 2,3-dioxygenase activity in the pregnant mouse has been reported to be a competitive inhibitor for the rabbit intestinal isoform of the enzyme. As shown in Fig. 1, 1-methyl-tryptophan is competitive with L-tryptophan for human placental indoleamine 2,3-dioxygenase. The Ki values giving half-maximal inhibition for L-tryptophan was found to be 68 µM for 1-methyl-DL-tryptophan [21].
Fig. 1.
Effect of 1-methyl-tryptophan on indoleamine 2,3-dioxygenase activity [21]. The rate of formation of the product catalysed by indoleamine 2,3-dioxygenase was determined over a 30 minutes period in a medium containing varying concentrations of 1-methyl-DL-tryptophan, 20 mM ascorbic acid, 10 µM methylene blue, 100 units mL-1 catalase and 50 mM potassium phosphate buffer (pH 6.5) in the presence of indicated concentrations of L-tryptophan. 1-Methyl-DL-tryptophan: •, 0 µM; ○, 40 µM; ▼, 200 µM; ▽, 400 µM. Inset is a replot for the apparent Km value (Kmapp) the inhibitor concentrations used to determined the Ki value; the line was determined by least-squares linear regression analysis. Data represent the mean±standard deviation of three separate experiments with triplicate assays.
These results indicate that the hypothesis of Munn et al. [4] that localised placental tryptophan catabolism prevents immunological fetal rejection in mouse is likely to be met for human pregnancy.
Regulation of human placental indoleamine 2,3-dioxygenase by interferon-γ
In a variety of tissues indoleamine 2,3-dioxygenase activity is known to be modulated by certain cytokines. Thus interferon-γ stimulates its expression in macrophages and monocytes [22]; other cytokines (e.g., interleukin-4) inhibit indoleamine 2,3-dioxygenase expression [23]. In order to clarify physiological significance of human placental indoleamine 2,3-dioxygenase, regulation of its expression by interferon-γ has been investigated using cultured human placental chorionic villi.
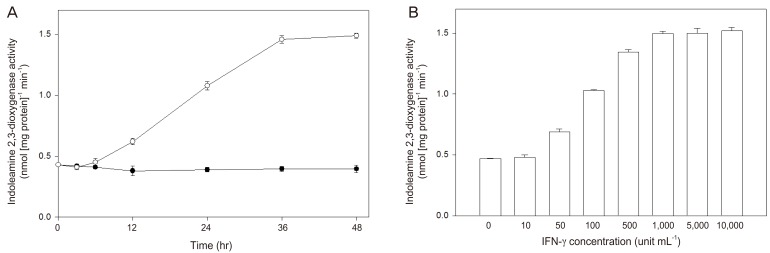
Human placental chorionic villi exposed to interferon-γ showed an increased indoleamine 2,3-dioxygenase activity in a time- and concentration-dependent manner (Fig. 2). The significant stimulatory effect of interferon-γ on indoleamine 2,3-dioxygenase activity could be detected after 12 hours of exposure of chorionic villi and was fully expressed 36 hours later, while the enhancement of mRNA expression began after 3 hours stimulation and reached a maximum at 12 hours [24]. Interferon-γ-stimulated indoleamine 2,3-dioxygenase mRNA expression was inhibited by interleukin-4 (Table 1), as was its activity by interleukin-4. Since interleukin-4 alone did not inhibit either mRNA level or enzyme activity, this effect may be due to the suppression of the increased transcription induced by interferon-γ. These data suggest that interleukin-4 may have a role in modulating indoleamine 2,3-dioxygenase availability in the human placenta.
Fig. 2.
Effect of interferon-γ (IFN-γ) on the stimulation of indoleamine 2,3-dioxygenase activity in cultured chorionic villi (From Kudo, et al. Mol Hum Reprod 2000;6:369-74, Oxford University Press [24]). Pieces of chorionic villi were cultured for the time indicated with 1,000 unit mL-1 IFN-γ or vehicle (phosphate-buffered saline) (A). Pieces of chorionic villi were cultured for 36 hours with the indicated concentrations of IFN-γ (B). Indoleamine 2,3-dioxygenase activity in tissue extract was determined. Data represent the mean±standard deviation of three separate experiments performed with three placentae.
Table 1.
Effect of interleukin-4 on interferon-γ-induced stimulation of indoleamine 2,3-dioxygenase mRNA expression in cultured chorionic villi (From Kudo, et al. Mol Hum Reprod 2000;6:369-74, Oxford University Press [24])
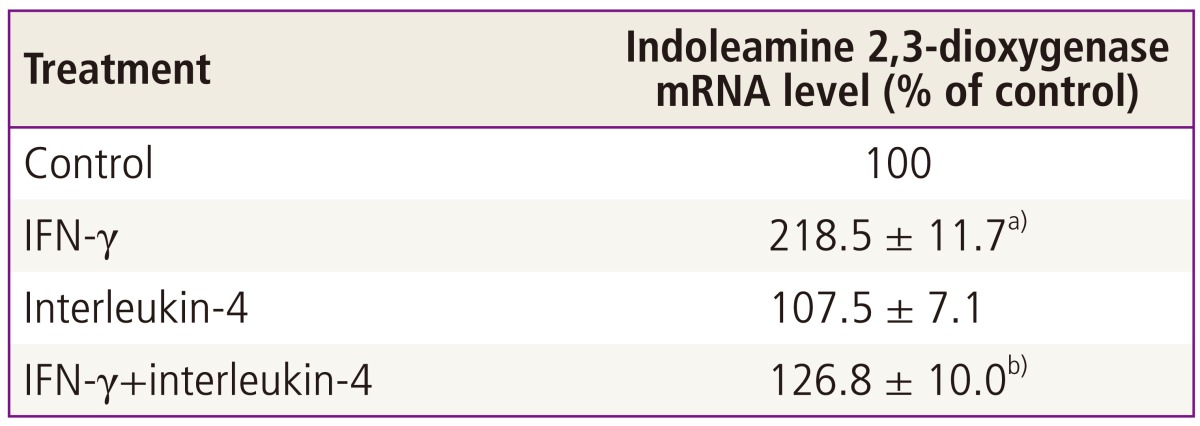
Pieces of chorionic villi were cultured with 1,000 unit mL-1 interferon-γ (IFN-γ) and/or 10 ng mL-1 interleukin-4 or vehicle (control) for 36 hours. The levels of indoleamine 2,3-dioxygenase messenger RNA (mRNA) and glyceraldehyde phosphate 3-dehydrogenase mRNA were analysed by reverse transcription-polymerase chain reaction. The intensity of either the indoleamine 2,3-dioxygenase or the glyceraldehydes phosphate 3-dehydrogenase band was quantitated and the ratio of the two was used as a normalised expression value of the indoleamine 2,3-dioxygenase gene. Values are mean±standard deviation of three separate experiments performed with three placentae, expressed as percentage of control (i.e., values cultured with vehicle alone).
a)Significantly different from control (P <0.001); b)Significantly different from IFN-γ (P <0.001).
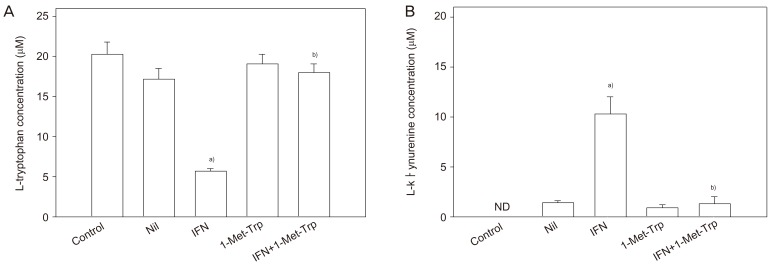
Concentrations of L-tryptophan and of L-kynurenine, the major indoleamine 2,3-dioxygenase degradation product, in the conditioned medium following in vitro culture of placental villous explants were determined (Fig. 3). The presence of interferon-γ in the culture medium stimulated L-tryptophan degradation markedly, consequently showing a marked increase in L-kynurenine concentration. The indoleamine 2,3-dioxygenase inhibitor 1-methyl-tryptophan showed inhibition of L-tryptophan catabolism in either the presence or absence of interferon-γ; both the decrease in L-tryptophan concentration and the associated increase in L-kynurenine concentration were reduced by the presence of 1-methyl-tryptophan [25].
Fig. 3.
Effect of interferon (IFN)-γ and 1-methyl-tryptophan on tryptophan catabolism by indoleamine 2,3-dioxygenase in placental explants [25]. Villous explants were cultured with or without 1,000 unit mL-1 IFN-γ and/or 2 mM 1-methyl-tryptophan (1-Met-Trp) or vehicle (Nil) for 36 hours. Concentrations of tryptophan (A) and kynurenine (B) in the conditioned medium were analysed by high-performance liquid chromatography. Values are mean±standard deviation of five separate experiments with triplicate assay from five different samples. Control, values without culture; ND, not detectable. a)Significantly different from Nil; b)Significantly different from IFN-γ.
Regulation of peripheral blood mononuclear cell proliferation by human placental indoleamine 2,3-dioxygenase mediated tryptophan degradation
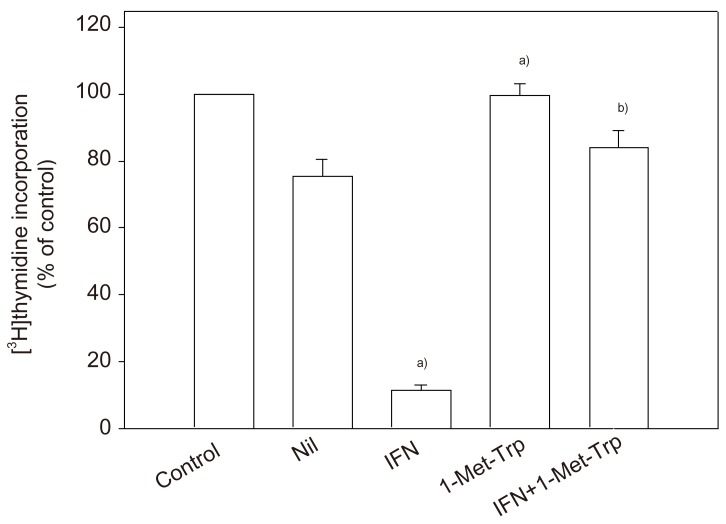
To clarify the physiological potential of indoleamine 2,3-dioxygenase in the human placenta, whether placental indoleamine 2,3-dioxygenase mediated tryptophan degradation regulates peripheral blood mononuclear cell proliferation was examined. For this, peripheral blood mononuclear cells were cultured in the same medium as that previously conditioned by culture of villous explants. Thymidine incorporation into DNA was then determined. The findings are that proliferation is inhibited by conditioned medium, that this inhibition is greater with medium conditioned in the presence of interferon-γ and that these effects are markedly blunted when 1-methyl-tryptophan was present during the previous conditioning with villous explants (Fig. 4). Return of a physiological concentration of L-tryptophan to the conditioned medium, but not of any of the other amino acids tested (including D-tryptophan) returned peripheral blood mononuclear cell proliferation rate to normal [26].
Fig. 4.
Peripheral blood mononuclear cell proliferation in medium previously conditioned by culture with villous explants [26]. Peripheral blood mononuclear cells were cultured for 72 hours either in non-conditioned medium or in medium previously conditioned by culture of explants with 1,000 unit mL-1 interferon-γ (IFN) and/or 2 mM 1-methyl-tryptophan (1-Met-Trp) for 36 hours. [3H]thymidine incorporation was then determined. Data represent the standard deviation of five separate experiments with quadruplicate assay, expressed as percentage of control (i.e., values cultured in non-conditioned medium). Control value for [3H]thymidine incorporation into DNA is (5.93±0.34)×104 cpm/well. Nil, vehicle. a)Significantly different from Nil; b)Significantly different from IFN-γ.
Tryptophan catabolism by placental indoleamine 2,3-dioxygenase in human pregnancy: a comparison of normal pregnancy and pre-eclampsia
It has been reported that plasma tryptophan levels decrease during normal pregnancy. Such a decrease may be related to immune activation phenomena during pregnancy. The maternal syndrome of pre-eclampsia is characterised by an excessive systemic inflammatory response induced by pregnancy [27]. Since the human placenta contains active indoleamine 2,3-dioxygenase, it is possible that in such pregnancies placental indoleamine 2,3-dioxygenase is less effective at controlling local and thus, indirectly, circulating tryptophan concentration. Therefore placental indoleamine 2,3-dioxygenase activity as well as indices of tryptophan catabolism have been studied in pregnant women, with or without pre-eclampsia, and non-pregnant women of reproductive age.
Table 2 summarise the results of high-performance liquid chromatography analysis of tryptophan and kynurenine concentrations in plasma. Tryptophan concentrations in plasma taken from both groups of pregnant women were significantly lower than those from women who were not pregnant. However plasma from women with normal pregnancy also had significantly lower concentrations than those from women with pre-eclampsia. Plasma kynurenine concentrations showed the converse patterns. Hence the ratios of indoleamine 2,3-dioxygenase product (plasma kynurenine) to substrate (plasma tryptophan), an index of tryptophan catabolism, were significantly increased in normal pregnant women compared either with women who were not pregnant or with women with pre-eclampsia [28].
Table 2.
Tryptophan and kynurenine concentrations and the ratio of kynurenine to tryptophan in plasma from women with pre-eclampsia or normal pregnancies and from non-pregnant women [28]
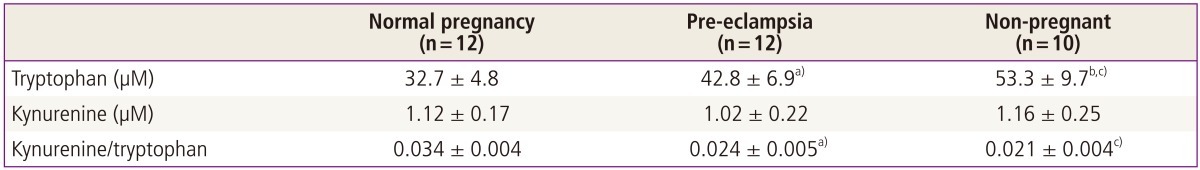
Concentrations of tryptophan and kynurenine in plasma were analysed by high-performance liquid chromatography. Values are given as mean ± standard deviation.
a)P <0.001 compared with normal pregnancy (Wilcoxon test); b)P <0.001 compared with pre-eclampsia (Mann-Whitney U test); c)p<0.001 compared with normal pregnancy (Mann-Whitney U test).
The indoleamine 2,3-dioxygenase activities in fresh placental villous tissue from pre-eclampsia were significantly lower when compared with those from normal pregnancy (Table 3). When villous tissue explants were cultured for 36 hours with or without interferon-γ, both indoleamine 2,3-dioxygenase activity and the percentage stimulation were still significantly lower in villous tissue from pre-eclampsia than was found for tissue from normal pregnancy [28].
Table 3.
Indoleamine 2,3-dioxygenase activity in placental villous tissue [28]
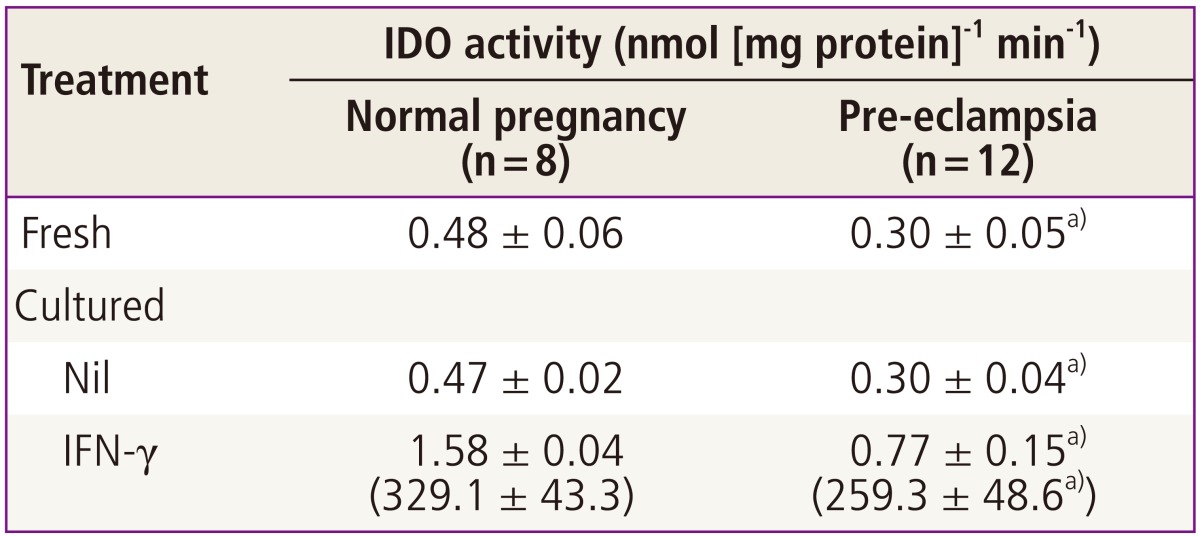
Villous explants were cultured with or without 1,000 unit mL-1 IFN-γ for 36 hours. IDO activity in the tissue extract of fresh or cultured villous explants was determined colorimetrically. Percentage stimulation is given in the parentheses. Values are mean± standard deviation of triplicate assays from indicated number of placentae.
IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-γ; Nil, vehicle.
a)Significantly different from normal pregnancy.
Conclusion
Experiments described in this review were conducted in order to investigate the physiological role of the tryptophan catabolising enzyme indoleamine 2,3-dioxygenase in the human placenta on the basis of Munn et al. [4]'s hypothesis in mouse that placental expression of this enzyme is crucial to prevent immunological rejection of the allogeneic fetus. Thus indoleamine 2,3-dioxygenase dependent localised depletion of tryptophan at the site of placentation has been proposed to be the mechanism of the suppression of maternal T cells which attack the conceptus [29].
That Munn et al.'s hypothesis in mouse is applicable to human pregnancy is suggested by the following pieces of evidence obtained from experiments in vitro described in this review. The human placenta expresses indoleamine 2,3-dioxygenase from early stages of pregnancy. 1-Methyl-tryptophan, the critical experimental tool used by Munn et al. to inhibit pregnant mouse indoleamine 2,3-dioxygenase, competitively inhibits the human placental enzyme (Fig. 1). Functional indoleamine 2,3-dioxygenase activity is also inhibited by 1-methyl-tryptophan in villous explants; when villous explants were cultured in the presence of 1-methyl-tryptophan, the normal decrease in tryptophan and increase in kynurenine concentrations in the conditioned medium were suppressed (Fig. 3). Indoleamine 2,3-dioxygenase mediated tryptophan degradation can inhibit peripheral blood mononuclear cell proliferation (Fig. 4); peripheral blood mononuclear cell proliferation following culture in the medium previously conditioned by culture of villous explants is markedly suppressed; inhibition of placental indoleamine 2,3-dioxygenase by 1-methyl-tryptophan during conditioning prevents inhibition of peripheral blood mononuclear cell proliferation. In addition to a potential role of indoleamine 2,3-dioxygenase, there are hypotheses suggesting roles for several other molecules or cells to explain maternal tolerance of the fetal allograft [30]. Tolerance of the fetal allograft is the results of the integration of numerous mechanisms of various origins and modes of action.
It has been suggested that the manifestations of the maternal syndrome of pre-eclampsia are not the results of processes distinct from those of normal pregnancy but simply the extreme end of the distribution of maternal intravascular inflammatory responses to normal pregnancy since activation of peripheral blood leukocytes is already well established in normal pregnancy [19,27]. Although the causes of these changes in peripheral blood leukocytes have not been defined, the evidence points to the placenta as the stimulus for pre-eclampsia [31,32]. The changes in the levels of cytokines observed in pre-eclampsia may be causally related to the decreased expression of placental indoleamine 2,3-dioxygenase since indoleamine 2,3-dioxygenase mediated tryptophan depletion can specifically suppress the proliferation of T helper lymphocytes which are a major source of cytokines. The increased circulating pro-inflammatory cytokines can activate maternal leukocytes and thus can develop exaggerated system inflammatory response in this condition. The cause of decreased expression level of indoleamine 2,3-dioxygenase in pre-eclamptic placenta remains to be defined. However, it is likely that increased plasma tryptophan concentration in women with pre-eclampsia and tryptophan dependent proliferation of lymphocytes indicate a causal relationship between suppressed indoleamine 2,3-dioxygenase expression and exaggerated inflammatory response in this maternal syndrome.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–338. [Google Scholar]
- 2.Bonney EA, Matzinger P. The maternal immune system's interaction with circulating fetal cells. J Immunol. 1997;158:40–47. [PubMed] [Google Scholar]
- 3.Hutter H, Hammer A, Dohr G, Hunt JS. HLA expression at the maternal-fetal interface. Dev Immunol. 1998;6:197–204. doi: 10.1155/1998/65065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida R, Hayaishi O. Indoleamine 2,3-dioxygenase. Methods Enzymol. 1987;142:188–195. doi: 10.1016/s0076-6879(87)42028-4. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem J. 1985;230:635–638. doi: 10.1042/bj2300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida R, Urade Y, Tokuda M, Hayaishi O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci U S A. 1979;76:4084–4086. doi: 10.1073/pnas.76.8.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai W, Pan H, Kwok O, Dubey JP. Human indoleamine 2,3-dioxygenase inhibits Toxoplasma gondii growth in fibroblast cells. J Interferon Res. 1994;14:313–317. doi: 10.1089/jir.1994.14.313. [DOI] [PubMed] [Google Scholar]
- 9.Dai W, Gupta SL. Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. Biochem Biophys Res Commun. 1990;168:1–8. doi: 10.1016/0006-291x(90)91666-g. [DOI] [PubMed] [Google Scholar]
- 10.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 11.Steckel NK, Kuhn U, Beelen DW, Elmaagacli AH. Indoleamine 2,3-dioxygenase expression in patients with acute graft-versus-host disease after allogeneic stem cell transplantation and in pregnant women: association with the induction of allogeneic immune tolerance? Scand J Immunol. 2003;57:185–191. doi: 10.1046/j.1365-3083.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 12.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 13.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 14.Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Semin Reprod Endocrinol. 1998;16:65–73. doi: 10.1055/s-2007-1016254. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 16.Barden A, Graham D, Beilin LJ, Ritchie J, Baker R, Walters BN, et al. Neutrophil CD11B expression and neutrophil activation in pre-eclampsia. Clin Sci (Lond) 1997;92:37–44. doi: 10.1042/cs0920037. [DOI] [PubMed] [Google Scholar]
- 17.Oian P, Omsjo I, Maltau JM, Osterud B. Increased sensitivity to thromboplastin synthesis in blood monocytes from pre-eclamptic patients. Br J Obstet Gynaecol. 1985;92:511–517. doi: 10.1111/j.1471-0528.1985.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 18.von Dadelszen P, Wilkins T, Redman CW. Maternal peripheral blood leukocytes in normal and pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1999;106:576–581. doi: 10.1111/j.1471-0528.1999.tb08327.x. [DOI] [PubMed] [Google Scholar]
- 19.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 20.Kudo Y, Boyd CA, Spyropoulou I, Redman CW, Takikawa O, Katsuki T, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61:87–98. doi: 10.1016/j.jri.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kudo Y, Boyd CA. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochim Biophys Acta. 2000;1500:119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 22.Takikawa O, Habara-Ohkubo A, Yoshida R. IFN-gamma is the inducer of indoleamine 2,3-dioxygenase in allografted tumor cells undergoing rejection. J Immunol. 1990;145:1246–1250. [PubMed] [Google Scholar]
- 23.Musso T, Gusella GL, Brooks A, Longo DL, Varesio L. Interleukin-4 inhibits indoleamine 2,3-dioxygenase expression in human monocytes. Blood. 1994;83:1408–1411. [PubMed] [Google Scholar]
- 24.Kudo Y, Boyd CA, Sargent IL, Redman CW. Modulation of indoleamine 2,3-dioxygenase by interferon-gamma in human placental chorionic villi. Mol Hum Reprod. 2000;6:369–374. doi: 10.1093/molehr/6.4.369. [DOI] [PubMed] [Google Scholar]
- 25.Kudo Y, Boyd CA. The role of L-tryptophan transport in L-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants. J Physiol. 2001;531:417–423. doi: 10.1111/j.1469-7793.2001.0417i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535:207–215. doi: 10.1111/j.1469-7793.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 28.Kudo Y, Boyd CA, Sargent IL, Redman CW. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188:719–726. doi: 10.1067/mob.2003.156. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thellin O, Coumans B, Zorzi W, Igout A, Heinen E. Tolerance to the foeto-placental 'graft': ten ways to support a child for nine months. Curr Opin Immunol. 2000;12:731–737. doi: 10.1016/s0952-7915(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 31.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]